Write the Formula Unit for the following Compounds Determining the formula for Magnesium Fluoride: Identify the charges, cross charges, subscript numbers, and common subscript such as MnO, FeO, which also includes MgO. Write Formula Unit For the Ionic Compounds Name Cation (+1) Anion (-1) Formula Sodium Chloride Na+ Cl- NaCl Aluminum Chloride Al3+ Cl- AlCl3 Aluminum Phosphide Al3+ P3- AlP Magnesium Oxide Mg2+ O2- MgO Cesium Fluoride Cs+ F- CsF Strontium Nitride Sr2+ N3- Sr3N2 Lithium Sulfide Li+ S2- Li2S Calcium Chloride Ca2+ Cl- CaCl2 Sodium Bromide Na+ Br- NaBr Beryllium Iodide Be2+ I- BeI2 Strontium Fluoride Sr2+ F- SrF2 Aluminum Fluoride Al3+ F- AlF3 Potassium Nitride K+ N3- KN Sodium Sulfide Na+ S2- Na2S Lithium Oxide Li+ O2- Li2O Calcium Oxide Ca2+ O2- CaO
The Correct Answer and Explanation is:
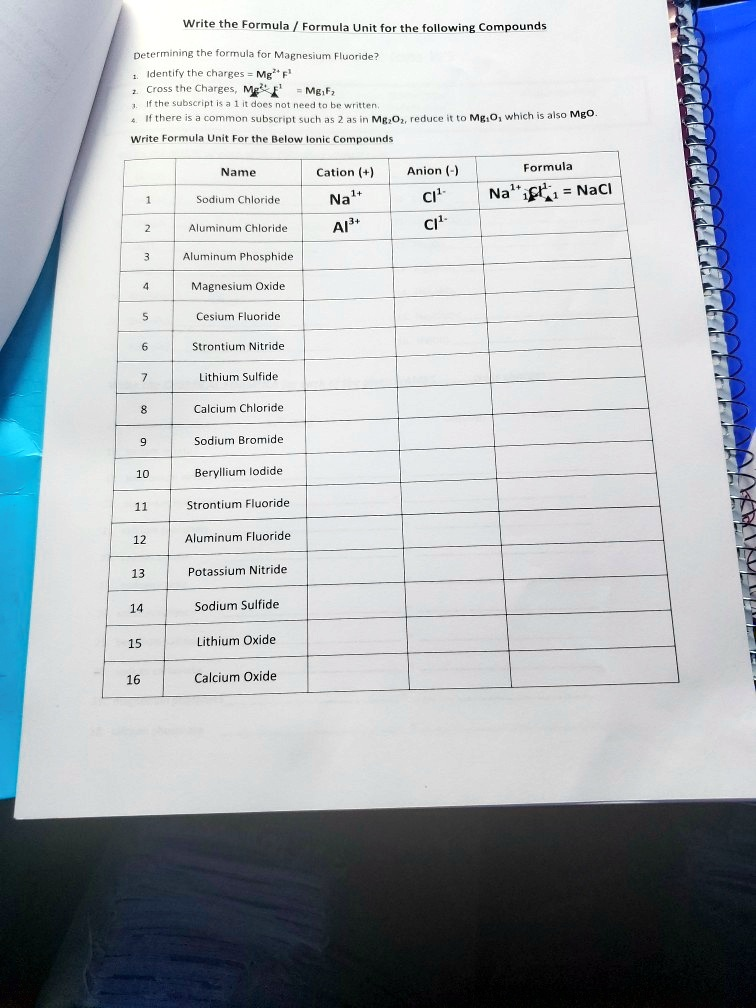
Completed Worksheet
| Name | Cation (+) | Anion (-) | Formula | |
| 1 | Sodium Chloride | Na¹⁺ | Cl¹⁻ | NaCl |
| 2 | Aluminum Chloride | Al³⁺ | Cl¹⁻ | AlCl₃ |
| 3 | Aluminum Phosphide | Al³⁺ | P³⁻ | AlP |
| 4 | Magnesium Oxide | Mg²⁺ | O²⁻ | MgO |
| 5 | Cesium Fluoride | Cs¹⁺ | F¹⁻ | CsF |
| 6 | Strontium Nitride | Sr²⁺ | N³⁻ | Sr₃N₂ |
| 7 | Lithium Sulfide | Li¹⁺ | S²⁻ | Li₂S |
| 8 | Calcium Chloride | Ca²⁺ | Cl¹⁻ | CaCl₂ |
| 9 | Sodium Bromide | Na¹⁺ | Br¹⁻ | NaBr |
| 10 | Beryllium Iodide | Be²⁺ | I¹⁻ | BeI₂ |
| 11 | Strontium Fluoride | Sr²⁺ | F¹⁻ | SrF₂ |
| 12 | Aluminum Fluoride | Al³⁺ | F¹⁻ | AlF₃ |
| 13 | Potassium Nitride | K¹⁺ | N³⁻ | K₃N |
| 14 | Sodium Sulfide | Na¹⁺ | S²⁻ | Na₂S |
| 15 | Lithium Oxide | Li¹⁺ | O²⁻ | Li₂O |
| 16 | Calcium Oxide | Ca²⁺ | O²⁻ | CaO |
Explanation
Writing the chemical formula for an ionic compound involves balancing the positive and negative charges of the ions to create a neutrally charged formula unit. An ionic compound is typically formed between a metal, which forms a positive ion (cation), and a nonmetal, which forms a negative ion (anion). The process follows a few logical steps.
First, you must identify the ions and their respective charges. For main group elements, the charge is often predictable from their position on the periodic table. For instance, metals in Group 1 (like Sodium, Lithium, Potassium) form ions with a +1 charge. Group 2 metals (like Magnesium, Calcium, Strontium) form ions with a +2 charge. Aluminum, in Group 13, typically forms a +3 ion. For nonmetals, Group 17 halogens (like Fluorine, Chlorine, Bromine) form anions with a -1 charge. Group 16 nonmetals (like Oxygen, Sulfur) form anions with a -2 charge, and Group 15 nonmetals (like Nitrogen, Phosphorus) form anions with a -3 charge.
Once you have the cation and anion with their charges, the goal is to find the simplest whole number ratio of ions that makes the total charge of the compound equal to zero. A helpful technique for this is the “crisscross” method, as shown on your worksheet. In this method, the numerical value of the cation’s charge becomes the subscript for the anion, and the numerical value of the anion’s charge becomes the subscript for the cation. For example, with Aluminum Chloride (Al³⁺ and Cl¹⁻), the 3 from aluminum crosses over to become the subscript for chlorine, and the 1 from chlorine crosses over to become the subscript for aluminum. This gives Al₁Cl₃.
Finally, there are two important rules for simplifying the formula. If a subscript is 1, it is omitted from the final formula, so Al₁Cl₃ becomes AlCl₃. Secondly, if the subscripts share a common factor, they must be reduced to their simplest ratio. For Magnesium Oxide (Mg²⁺ and O²⁻), the crisscross method initially gives Mg₂O₂. Since both subscripts can be divided by 2, the formula is reduced to its simplest form, which is MgO. This reflects that one magnesium ion (+2) perfectly balances one oxide ion (-2). Following these steps consistently will allow you to correctly determine the formula for any binary ionic compound
