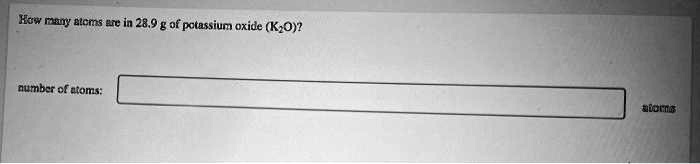
How many atoms are in 28.9 g of potassium oxide (
)? number of atoms:
The Correct Answer and Explanation is:
Answer: 5.54 x 10²³ atoms
Explanation:
To determine the total number of atoms in 28.9 grams of potassium oxide (K₂O), we follow a multi-step process that converts mass into the number of individual atoms. This process involves using the molar mass of the compound and Avogadro’s number.
First, we must calculate the molar mass of potassium oxide (K₂O). This is the sum of the atomic masses of all atoms in one molecule of the compound. Using the periodic table, the atomic mass of potassium (K) is approximately 39.10 g/mol , and the atomic mass of oxygen (O) is approximately 16.00 g/mol . Since the formula is K₂O, we have two potassium atoms and one oxygen atom. The molar mass is calculated as (2 × 39.10 g/mol ) + (1 × 16.00 g/mol ), which equals 94.20 g/mol .
Next, we convert the given mass of K₂O into moles. A mole is a unit that represents a specific quantity of a substance. We can find the number of moles by dividing the given mass by the molar mass:
Moles of K₂O = 28.9 g / 94.20 g/mol ≈ 0.307 moles of K₂O.
Now we can determine how many individual formula units (molecules) of K₂O are in this sample. We use Avogadro’s number, which is 6.022 × 10²³, the number of particles (atoms, molecules, etc.) in one mole of a substance.
Number of K₂O molecules = 0.307 moles × (6.022 × 10²³ molecules/mole) ≈ 1.85 × 10²³ molecules.
Finally, we find the total number of atoms. The chemical formula K₂O shows that each molecule contains 2 potassium atoms and 1 oxygen atom, for a total of 3 atoms per molecule. To find the total number of atoms in the sample, we multiply the number of molecules by the number of atoms per molecule:
Total atoms = (1.85 × 10²³ molecules) × (3 atoms/molecule) ≈ 5.54 × 10²³ atoms.thumb_upthumb_down
