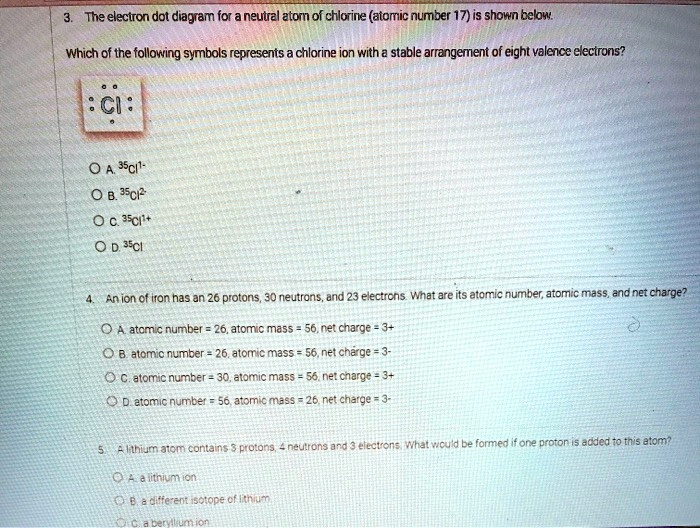
The electron dot diagram for a neutral atom of chlorine (atomic number 17) is shown below. Which of the following symbols represents a chlorine ion with a stable arrangement of eight valence electrons? CI = A. Cl1- B. C1+ C. 3CI+ D. Cl An ion of iron has 26 protons, 30 neutrons, and 23 electrons. What are its atomic number, atomic mass, and net charge? Atomic number = 26, atomic mass = 56, net charge = 0
The Correct Answer and Explanation is:
Of the options provided, the correct answer is A. ³⁵Cl¹⁻.
Here is a detailed explanation:
The question asks to identify the correct symbol for a chlorine ion with a stable arrangement of eight valence electrons. The provided electron dot diagram for a neutral chlorine atom shows the symbol “Cl” surrounded by seven dots. These dots represent the seven valence electrons in the outermost energy level of a neutral chlorine atom.
Atoms tend to achieve a stable electron configuration, which usually means having a full outer shell of electrons. For many elements, including chlorine, this stable state consists of eight valence electrons, a principle known as the octet rule.
To achieve a stable octet, the chlorine atom, which starts with seven valence electrons, needs to acquire one additional electron. Gaining an electron is energetically more favorable for chlorine than losing all seven of its valence electrons.
When a neutral atom gains an electron, it becomes a negatively charged ion, called an anion. A neutral atom has an equal number of protons (positive charges) and electrons (negative charges). A neutral chlorine atom (atomic number 17) has 17 protons and 17 electrons, resulting in a net charge of zero. When it gains one electron to complete its octet, it will have 17 protons and 18 electrons. The net charge is calculated as the number of protons minus the number of electrons: 17 – 18 = -1.
Therefore, the resulting stable ion has a charge of 1-. This ion is called the chloride ion, and its symbol is written as Cl¹⁻ or simply Cl⁻. The options include the mass number 35, which specifies a particular isotope of chlorine but does not change the process of ion formation. Combining the mass number, the element symbol, and the charge gives the complete symbol ³⁵Cl¹⁻. This symbol correctly represents a chlorine ion that has gained one electron to achieve a stable octet of eight valence electrons.
