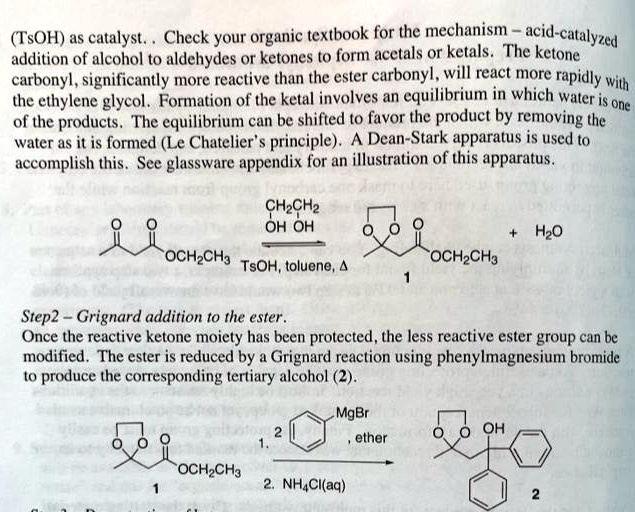
In the multistep synthesis preparation of 4,4-Diphenyl-3-buten-2-one, the first step involves the protection of the ketone carbonyl group so that the less reactive ester carbonyl can instead be reduced during the Grignard addition. What product will be created if the first step was skipped and therefore the ketone carbonyl group was not protected? Please write the new mechanism when the first step is skipped. I have attached the mechanism for how Step 1 and 2 will look when no step is skipped. Water is formed during the reaction and is removed using a Dean-Stark apparatus, following Le Chatelier’s principle. A glassware appendix is provided for an illustration of this apparatus. CHCH2OH OH + HO OCH2CH3 OCHCHgTsOH, toluene, Step 2 – Grignard addition to the ester: Once the reactive ketone moiety has been protected, the less reactive ester group can be modified. The ester is reduced by a Grignard reaction using phenylmagnesium bromide to produce the corresponding tertiary alcohol. MgBr, ether OH OCHCH3 2. NH4Cl(aq)
The Correct Answer and Explanation is:
In the scenario where the first step (protection of the ketone carbonyl group) is skipped, the Grignard reagent, phenylmagnesium bromide (PhMgBr), will react with the more reactive ketone group rather than the ester group. This would lead to the formation of a tertiary alcohol from the ketone carbonyl group rather than the desired modification of the ester.
Here’s how the mechanism would proceed without the protection step:
- Grignard Addition: The phenylmagnesium bromide (PhMgBr) will attack the carbonyl carbon of the ketone, leading to the formation of a tetrahedral alkoxide intermediate. The oxygen of the carbonyl group, which was previously double-bonded, now has a negative charge due to the nucleophilic attack.
- Protonation: After the alkoxide intermediate is formed, the protonation of this intermediate occurs when the reaction mixture is treated with water (NH4Cl(aq) is typically used for this step). This protonation results in the formation of a tertiary alcohol with two phenyl groups attached to the central carbon.
- Final Product: The result is the formation of a tertiary alcohol instead of the desired product, which should have involved the reduction of the ester group (Step 2). The reaction of the Grignard reagent with the ketone group instead of the ester will prevent the subsequent desired product formation. The formation of the tertiary alcohol would prevent the creation of the desired 4,4-diphenyl-3-buten-2-one.
The key takeaway here is that skipping the protection of the ketone group leads to the Grignard reagent attacking the more reactive ketone rather than the ester, resulting in a different product (a tertiary alcohol) and not the intended 4,4-Diphenyl-3-buten-2-one.
