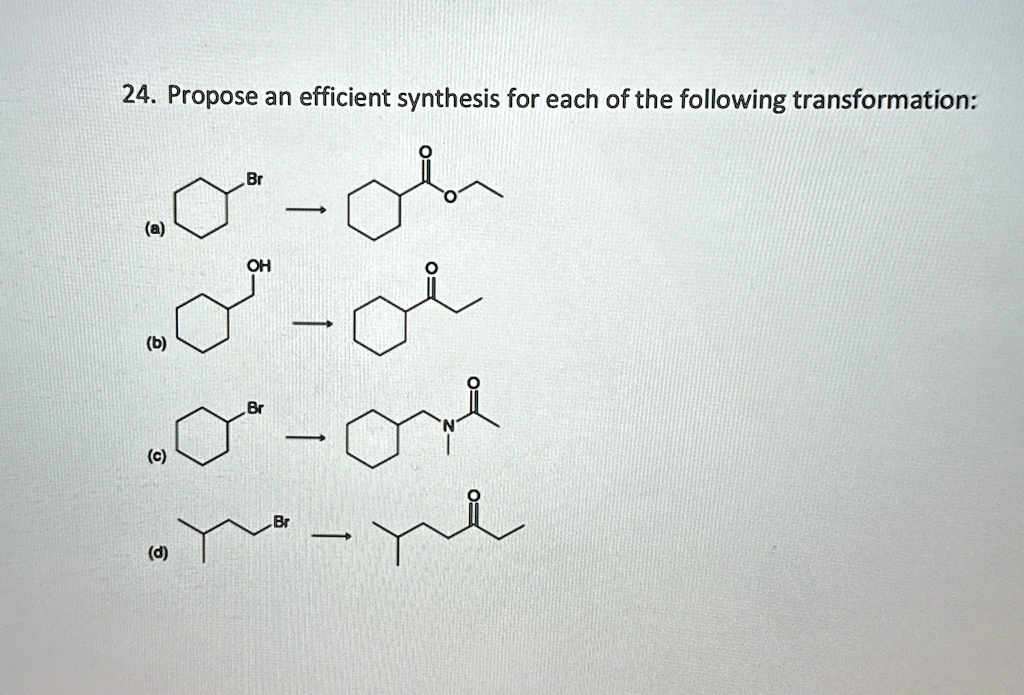
Propose an efficient synthesis for each of the following transformation: Br O (a) —–> OH (b) —–> Br (c) —–> Br (d) —
The Correct Answer and Explanation is:
Based on the transformations depicted in the image, here is a concise synthetic route for each:
(a) Br → CO₂Me (methyl ester) Reaction Sequence:
- Mg, dry ether → Grignard reagent (R–MgBr)
- CO₂ (dry ice), then dilute HCl → Carboxylic acid
- CH₃OH, H⁺ (acid-catalyzed esterification) → Methyl ester
(b) OH → C=O (ketone) Reaction Sequence:
- PCC (pyridinium chlorochromate) or DMP (Dess-Martin periodinane) in CH₂Cl₂ → Ketone
(c) Br → CONH₂ (amide) Reaction Sequence:
- NaCN, ethanol → Nitrile (R–CN)
- Acidic or basic hydrolysis (e.g., H₃O⁺, heat) → Amide (CONH₂)
(d) Br (on primary carbon) → C=O (ketone) Reaction Sequence:
- NaCN → Nitrile
- Grignard reagent (MeMgBr) → Imine intermediate
- Acidic hydrolysis → Ketone (via ketimine)
Explanation Each route employs classic synthetic strategies aligned with the functional group interconversions in organic chemistry.
In (a), the bromine substituent on cyclohexane enables nucleophilic substitution to generate a Grignard reagent. Reaction with carbon dioxide introduces a carboxylic acid, which is then esterified under acidic methanol conditions to form the methyl ester.
For (b), secondary alcohols on a cyclohexane ring are cleanly oxidized to ketones using mild oxidizing agents like PCC or DMP. These reagents avoid overoxidation to carboxylic acids, preserving the desired ketone product.
The conversion in (c) begins with substitution of bromine by cyanide ion to introduce a nitrile group. Controlled hydrolysis under acidic or basic conditions then transforms the nitrile into an amide, a key functional group in biochemical systems and polymers.
In (d), starting from a bromoalkane, the route begins by converting the alkyl halide into a nitrile. Treatment with a Grignard reagent adds a methyl group, forming a ketimine intermediate. Hydrolysis in acidic conditions finalizes the transformation into a ketone.
Each strategy emphasizes selectivity, functional group compatibility, and accessible reagents, ensuring overall efficiency in synthesis.
