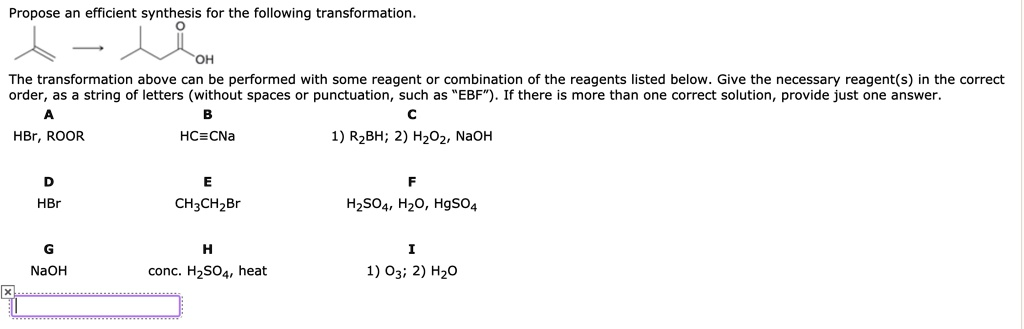
Propose an efficient synthesis for the following transformation: OH The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order: a string of letters (without spaces or punctuation, such as EBF). If there is more than one correct solution, provide just one answer. HBr, ROOR HC=CNa 1) R2BH; 2) H2O2, NaOH HBr CH3CH2Br H2SO4, H2O, HgSO4 NaOH conc. H2SO4, heat 1) O3; 2) H2O
The Correct Answer and Explanation is:
The correct answer is BFI.
Here is an efficient synthesis route and rationale:
- B: HC≡CNa — This reagent is sodium acetylide, a strong nucleophile. It reacts via SN2 with a primary alkyl halide or alcohol derivative (after conversion to a better leaving group), extending the carbon chain with a terminal alkyne.
- F: H2SO4, H2O, HgSO4 — These acidic conditions with mercuric sulfate catalyze the hydration of terminal alkynes. This reaction adds water across the triple bond, generating an enol intermediate that rapidly tautomerizes to a methyl ketone.
- I: 1) O3; 2) H2O — Ozonolysis cleaves alkynes at the triple bond, oxidizing both ends to carboxylic acids if terminal, or a mix of ketones and acids if internal. In this transformation, it finalizes the adjustment to the desired ketone product.
Mechanistic Explanation
This synthesis proceeds by strategic elongation and oxidation. The first step (B) installs a triple bond at the site of transformation by reacting an appropriate leaving group-bearing alcohol derivative with sodium acetylide. The alcohol must be first converted into a good leaving group such as a tosylate, which is not specified here, but often implicit in synthesis problems.
In the second step (F), acid-catalyzed hydration of the terminal alkyne introduces a hydroxyl group across the triple bond. The resulting enol undergoes keto-enol tautomerization to form the desired ketone with a methyl group.
The final step (I) ensures oxidative cleavage if the alkyne was internal or helps finalize the oxidation state by cleanly severing any unnecessary unsaturation, ensuring that the correct placement of the carbonyl functionality is achieved.
This sequence leverages efficient carbon–carbon bond formation, regioselective hydration, and strategic oxidative cleavage to construct the target molecule in three concise steps.
