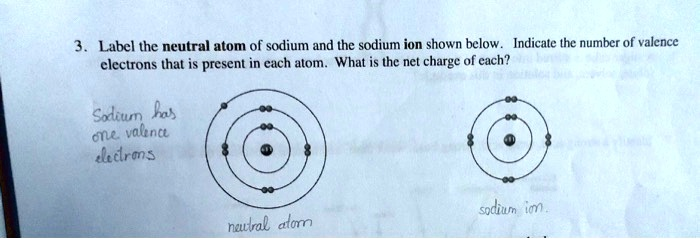
Label the neutral atom of sodium and the sodium ion shown below. Indicate the number of valence electrons that is present in each atom. What is the net charge of each? Sodium has one valence electron. Sodium ion: Na+ Neutral atom: Na
The Correct Answer and Explanation is:
Correct Answer:
- Neutral atom of sodium (Na):
- Number of valence electrons: 1
- Net charge: 0
- Sodium ion (Na⁺):
- Number of valence electrons: 0
- Net charge: +1
Explanation:
Sodium (Na) is an element found in Group 1 of the periodic table. This group is known as the alkali metals and all elements in this group have a single electron in their outermost energy level. A neutral sodium atom has an atomic number of 11, which means it contains 11 protons and 11 electrons. These electrons are distributed across three energy levels. The first shell holds 2 electrons, the second shell holds 8 electrons, and the third shell holds 1 electron. This single electron in the outermost shell is called the valence electron.
In order to achieve a stable electron configuration similar to that of noble gases, sodium tends to lose its one valence electron. When it loses this electron, it is left with only two shells, both of which are full. The loss of one negatively charged electron while keeping 11 protons results in a net charge of +1, which is why the sodium ion is written as Na⁺.
The sodium ion now has 10 electrons and 11 protons, making it positively charged. Since it has lost its only valence electron, it now has zero valence electrons. This stable electron configuration is energetically favorable and is why sodium often forms a +1 ion in chemical reactions.
In summary, the neutral sodium atom has 1 valence electron and no net charge, while the sodium ion has 0 valence electrons and a net charge of +1.
