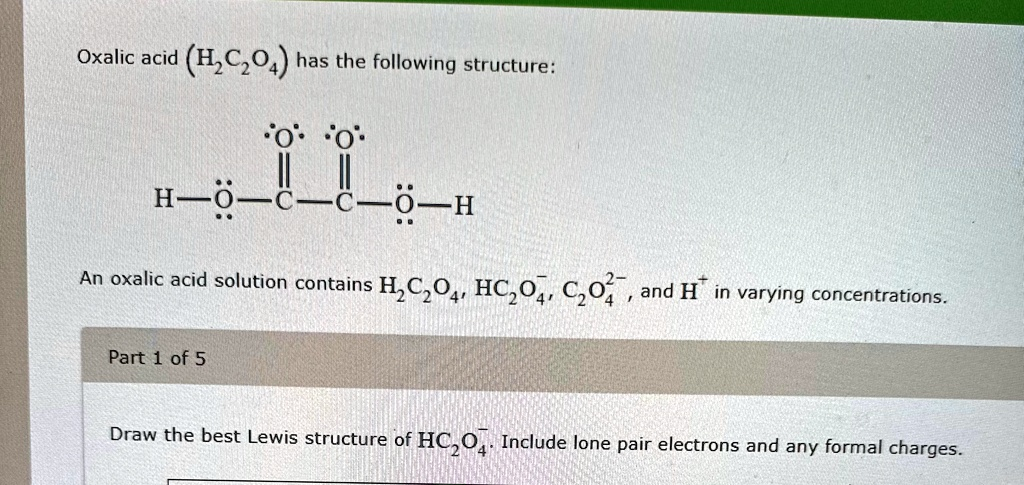
Oxalic acid (H2C2O4) has the following structure
The Correct Answer and Explanation is:
To draw the best Lewis structure of the hydrogen oxalate ion (HC₂O₄⁻), follow these steps:
Correct Lewis Structure of HC₂O₄⁻:
- The ion consists of a central carbon-carbon bond, with each carbon double bonded to one oxygen atom and single bonded to another oxygen atom.
- One hydrogen is bonded to a singly bonded oxygen (making it –OH).
- The extra negative charge is delocalized, usually represented on one of the singly bonded oxygen atoms.
- The carbon atoms are bonded together with a single bond (C–C).
- Include lone pairs around oxygen atoms to complete their octets.
Here is how the Lewis structure looks:
HO–C(=O)–C(=O)–O⁻
Now let’s break down the explanation.
Explanation
The HC₂O₄⁻ ion, also known as the hydrogen oxalate or binoxalate ion, is derived from oxalic acid (H₂C₂O₄), a diprotic acid. In the hydrogen oxalate form, only one hydrogen has been lost as a proton (H⁺), leaving the other hydrogen still bound to the molecule.
We start by calculating the total valence electrons:
- Two carbon atoms: 2 × 4 = 8 electrons
- Four oxygen atoms: 4 × 6 = 24 electrons
- One hydrogen atom: 1 electron
- One extra electron from the negative charge: +1 electron Total = 34 electrons
The structure needs to satisfy the octet rule for each atom except hydrogen, which needs only two electrons. Each carbon forms a double bond with one oxygen and a single bond with another oxygen. One of the singly bonded oxygens holds a negative charge and has three lone pairs. The –OH group contains hydrogen bonded to an oxygen, which has two lone pairs.
Formal charges help identify the most stable configuration. The negatively charged oxygen should be one that is singly bonded and not bearing hydrogen. This allows the negative charge to be stabilized through resonance across the oxygen atoms.
The HC₂O₄⁻ ion can resonate through various Lewis structures where the negative charge is distributed over the singly bonded oxygens, giving it some delocalized character. This resonance contributes to the stability and acidity of the compound.
