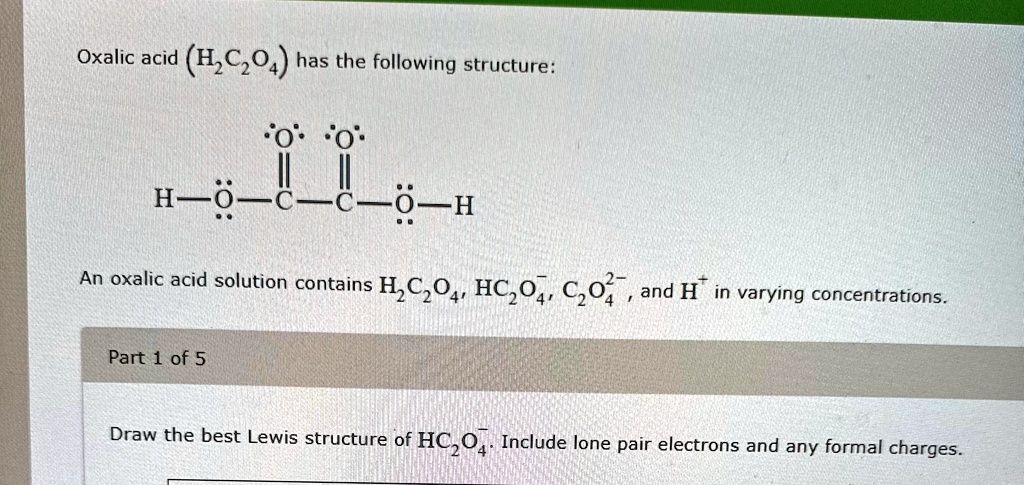
The Correct Answer and Explanation is:
To draw the best Lewis structure of the hydrogen oxalate ion (HC₂O₄⁻), we begin by analyzing the molecular structure.
Correct Lewis Structure of HC₂O₄⁻:
The ion consists of two carbon atoms connected by a single bond. Each carbon atom is doubly bonded to one oxygen atom and singly bonded to another. One of the singly bonded oxygens carries a negative formal charge, and one is protonated (–OH). The negative charge is delocalized across the ion, contributing to resonance.
Here’s a summary of the bonding:
- One carbon forms a double bond with one oxygen (C=O), and a single bond with an –OH group.
- The second carbon forms a double bond with an oxygen and a single bond with an O⁻ group.
- The two carbon atoms are connected by a single bond.
Each oxygen should have enough lone pairs to satisfy the octet rule, typically two or three lone pairs depending on whether the oxygen is involved in single or double bonds.
Explanation
The hydrogen oxalate ion, HC₂O₄⁻, is an intermediate species in the dissociation of oxalic acid. Constructing its Lewis structure requires accounting for 33 valence electrons: 1 from H, 8 from the two carbons, 24 from the four oxygens, and 1 additional electron for the negative charge.
We begin by connecting the atoms in a likely skeletal structure: C–C backbone with each carbon bonded to two oxygens. Placing double bonds with one oxygen on each carbon helps satisfy the octet rule and formal charges. The –OH group is formed by attaching a hydrogen atom to one of the singly bonded oxygens. The negative formal charge typically resides on the singly bonded oxygen without hydrogen because this arrangement minimizes charge separation and is most stable.
Each oxygen gets lone pairs to complete its octet, and formal charges are evaluated. The structure should show one negative charge and should be consistent with known resonance possibilities. This means there are contributing structures where the double bonds and the negative charge can shift positions among the oxygen atoms, enhancing stability via resonance.
This representation not only satisfies electron count and formal charge distribution but also reflects the resonance stability of the anion, making it the most appropriate Lewis structure for HC₂O₄⁻.
