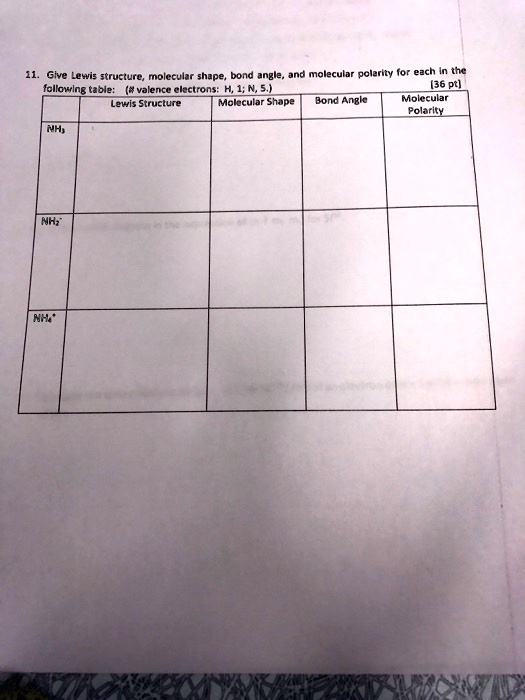
- Give Lewis structure, molecular shape, bond angle, and molecular polarity for each in the following table: (# valence electrons: H, 1; N, 5.) Lewis Structure Molecular Shape Bond Angle Molecular Polarity NH
NH
NH
The Correct Answer and Explanation is:
Here’s a filled-out summary table for the three nitrogen-hydrogen species:
| Species | Lewis Structure | Molecular Shape | Bond Angle | Molecular Polarity |
|---|---|---|---|---|
| NH₃ | N with 3 single bonds to H, 1 lone pair | Trigonal pyramidal | ~107° | Polar |
| NH₂⁻ | N with 2 single bonds to H, 2 lone pairs, 1 extra electron | Bent or angular | ~104.5° | Polar |
| NH₄⁺ | N with 4 single bonds to H, no lone pairs | Tetrahedral | 109.5° | Nonpolar |
Explanation:
The Lewis structure represents how valence electrons are arranged in a molecule. Nitrogen has five valence electrons. In ammonia (NH₃), three of these form covalent bonds with hydrogen atoms while the remaining two form a lone pair. This lone pair repels the bonded electrons, creating a trigonal pyramidal shape. The bond angle is slightly less than the ideal tetrahedral angle because of the lone pair’s stronger repulsion, reducing it to about 107 degrees. The molecule is polar since the lone pair introduces asymmetry and nitrogen is more electronegative than hydrogen, pulling electron density toward itself.
For the amide ion (NH₂⁻), nitrogen forms two single bonds with hydrogen and carries two lone pairs plus an additional electron for the negative charge. This arrangement gives a bent geometry similar to water, with bond angles around 104.5 degrees. The lone pairs again push the bonded atoms closer together. Due to asymmetrical shape and unequal electron sharing, NH₂⁻ is polar.
Ammonium ion (NH₄⁺) is formed when nitrogen donates one electron to bond with a fourth hydrogen atom and carries a positive charge. With four equivalent N–H bonds and no lone pairs, the molecule assumes a tetrahedral shape with bond angles of 109.5 degrees. Since the molecule is symmetrical and all dipole moments cancel out, it is nonpolar.
This analysis highlights how electron domain geometry shapes molecular behavior and polarity.
