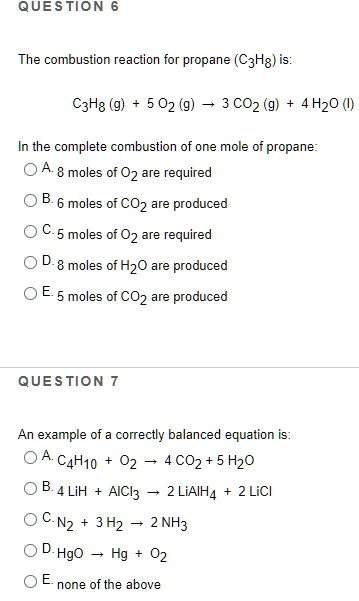
The combustion reaction for propane
The Correct Answer and Explanation is:
Here are the correct answers followed by a detailed explanation:
Question 6 Answer: C. 5 moles of O₂ are required Question 7 Answer: C. N₂ + 3 H₂ → 2 NH₃
Explanation
For Question 6, we are analyzing the complete combustion of propane, which is represented by the balanced equation:
C₃H₈ (g) + 5 O₂ (g) → 3 CO₂ (g) + 4 H₂O (l)
The coefficients in this chemical equation provide the molar ratios. They indicate that one mole of propane (C₃H₈) reacts with five moles of oxygen gas (O₂) to produce three moles of carbon dioxide (CO₂) and four moles of water (H₂O). This reaction exemplifies a typical combustion process, which involves a hydrocarbon reacting with oxygen to form carbon dioxide and water. Therefore, the correct number of moles of oxygen required to completely combust one mole of propane is five. Option C correctly reflects this stoichiometric ratio.
For Question 7, we are identifying the only correctly balanced chemical equation. Option C:
N₂ + 3 H₂ → 2 NH₃
This equation is balanced because it contains equal numbers of atoms of each element on both sides. On the left, there are two nitrogen atoms and six hydrogen atoms. On the right, two molecules of ammonia (NH₃) each contain one nitrogen and three hydrogen atoms, totaling two nitrogen atoms and six hydrogen atoms. This satisfies the law of conservation of mass. The other options either misrepresent the number of atoms or require adjustment in coefficients to achieve balance. Thus, Option C stands as the correct choice due to its accurate reflection of reactant-product equivalence in a chemical reaction.
