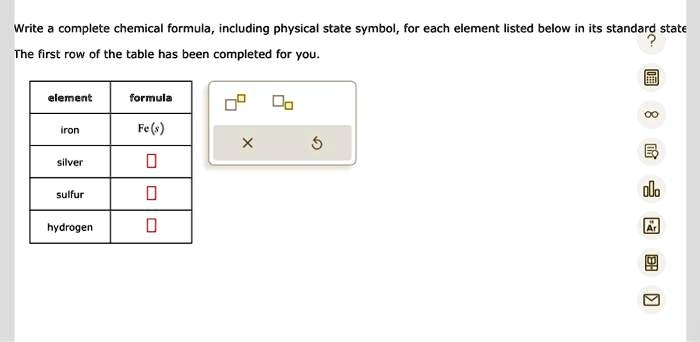
Write a complete chemical formula, including physical state symbol, for each element listed below in its standard state The first row of the table has been completed for you. element formula iron Fe(s) silver sulfur hydrogen
The Correct Answer and Explanation is:
Here’s the completed table showing each element in its standard state:
| Element | Formula |
|---|---|
| Iron | Fe(s) |
| Silver | Ag(s) |
| Sulfur | S(s) |
| Hydrogen | H₂(g) |
Explanation:
The standard state of an element refers to its most stable physical form at 25 degrees Celsius and 1 atmosphere of pressure. This information is essential when writing balanced chemical equations because it provides the baseline for enthalpy and Gibbs free energy calculations.
- Iron (Fe): This is already provided. Iron exists as a lustrous, silvery-gray metal in its standard form and is solid at room temperature, hence the formula Fe(s).
- Silver (Ag): Like iron, silver is a metal. At standard conditions, it is a solid with a shiny, metallic appearance. Its formula is Ag(s), where (s) represents the solid state.
- Sulfur (S): Sulfur exists as a yellow crystalline solid in its most stable form at room temperature. It is not diatomic or polyatomic in elemental form under these conditions. The appropriate formula is S(s).
- Hydrogen (H₂): Hydrogen is unique compared to the other elements listed. In its standard state, hydrogen exists as a diatomic gas composed of two hydrogen atoms. This occurs because a single hydrogen atom is highly reactive and rarely exists independently under normal conditions. Its formula is H₂(g), where (g) represents the gaseous state.
Recognizing the standard physical states of elements supports accurate stoichiometric calculations and thermodynamic predictions. Additionally, it reinforces an understanding of molecular stability, bonding tendencies, and periodic trends such as metallic character and nonmetal reactivity.
