Draw a Lewis structure for SO2
in which all atoms obey the octet rule. Show formal charges. Do not consider ringed structures.
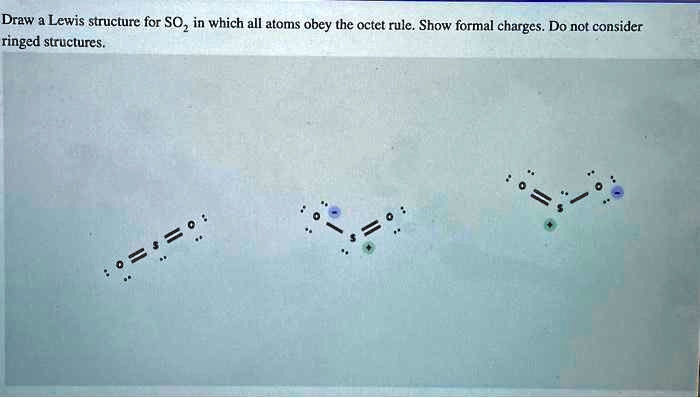
To correctly draw a Lewis structure for sulfur dioxide (SO₂) in which all atoms obey the octet rule and formal charges are shown, let us analyze the given options in your image. There are three Lewis structures shown. We will evaluate them and select the correct one.
Step-by-Step Analysis:
- Total Valence Electrons:
- Sulfur (S) has 6 valence electrons.
- Oxygen (O) has 6 valence electrons.
- SO₂ has 1 sulfur and 2 oxygen atoms:
→ 6 (S) + 6×2 (O) = 18 valence electrons total.
- Octet Rule:
Each atom (S and O) should have 8 electrons around it (shared and lone pairs). - Formal Charges:
We calculate formal charges to find the most stable structure. The formula is: Formal charge=Valence electrons−(Lone pair electrons + ½ Bonding electrons)\text{Formal charge} = \text{Valence electrons} – \text{(Lone pair electrons + ½ Bonding electrons)}Formal charge=Valence electrons−(Lone pair electrons + ½ Bonding electrons)
Structure Evaluation:
From the three structures shown in the image:
- Left structure: Both S=O bonds with no lone pair on sulfur.
- Sulfur has 4 bonding pairs (8 electrons total), so octet is satisfied.
- But if you calculate formal charges, both O atoms have 0 and sulfur has +2, which is less favorable.
- Middle structure: One S=O double bond and one S–O single bond with a negative formal charge on one oxygen and a positive charge on sulfur.
- Formal charges:
- Sulfur: +1
- Single-bonded O: –1
- Double-bonded O: 0
- This adds up to a net formal charge of 0, and all atoms follow the octet rule.
- This is the correct and most stable structure.
- Formal charges:
- Right structure: Similar to the middle one but the position of the double bond is switched.
- This is a resonance structure of the middle one.
- It is equally correct and contributes to the resonance hybrid.
Final Answer:
✅ The middle and right structures are correct Lewis structures for SO₂.
They follow the octet rule and show proper formal charges (–1 on one O, +1 on S).
These two are resonance structures of SO₂, and the true structure is a resonance hybrid of both.
