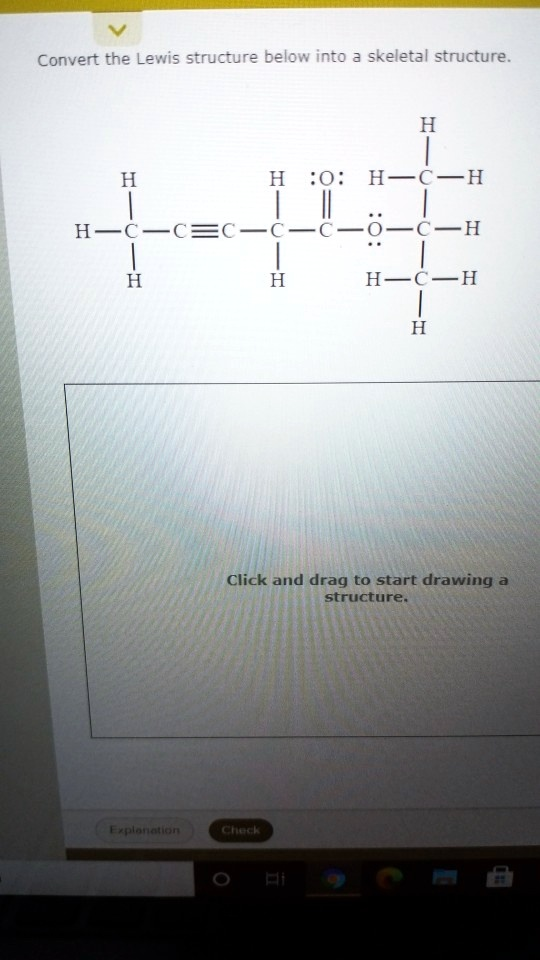
Convert the Lewis structure below into a skeletal structure. H H H:O: H-C-H H-C-C?C-C-C-O-C-H H H H-C-H H Click and drag to start drawing a structure. Explanation Check
The Correct Answer and Explanation is:
To convert the given Lewis structure into a skeletal (line-angle) structure, we first need to identify the carbon backbone and functional groups while omitting the hydrogen atoms bonded to carbon, as skeletal structures typically do.
Step-by-Step Breakdown:
1. Analyze the Lewis structure:
From left to right:
- A CH₃ (methyl) group is bonded to a CH group.
- The CH group is triple-bonded to a C, which is bonded to another CH₂.
- That CH₂ is bonded to a C=O (carbonyl) group.
- The carbonyl carbon is also bonded to an O which is bonded to a CH₂, and that continues with two more CH₂ groups.
So the molecule is:
CH₃–C≡C–CH₂–COO–CH₂–CH₂–CH₃
This corresponds to methyl butynoate extended with a propyl group on the ester oxygen.
Skeletal Structure Description:
- The carbon atoms are represented as vertices or ends of lines.
- Hydrogen atoms bonded to carbon are not shown.
- Heteroatoms like O and functional groups like carbonyls are shown explicitly.
The structure will look like this in skeletal format:
- Start with a line segment (representing CH₃–CH)
- Triple bond to next carbon (C≡C)
- Single bond to CH₂ (line extension)
- Then to carbonyl (C=O) and an oxygen
- Then continue to the chain of 3 carbons (O–CH₂–CH₂–CH₃)
So, the correct skeletal structure is a zigzag starting with a triple bond, followed by a carboxylic ester group, ending in a three-carbon chain (propyl group).
Explanation
A skeletal structure is a simplified representation of an organic molecule. It shows the carbon-carbon framework as a series of lines. Each point (or vertex) represents a carbon atom, and hydrogen atoms attached to carbon are generally omitted unless they are part of a functional group or directly bonded to atoms like oxygen or nitrogen. This style provides a cleaner, easier-to-read format for complex molecules.
In the given Lewis structure, we begin with a methyl group (CH₃) bonded to a carbon that shares a triple bond with another carbon. This arrangement forms a propyne-like fragment. Following this is a methylene (CH₂) group attached to a carbon double-bonded to an oxygen atom, forming a carbonyl. This indicates the presence of an ester or carboxylic acid group.
The carbonyl carbon is also single-bonded to an oxygen, which in turn is bonded to a three-carbon straight chain. This entire group is known as a propyl ester. Therefore, the molecule contains an ester functional group linking the alkyne portion on one side and a propyl group on the other.
In skeletal format, the triple bond is shown as three lines between two points. The ester is shown with a double bond to oxygen and a single bond to another oxygen leading to a propyl chain. These functional groups are essential because they determine the molecule’s chemical properties and reactivity.
This skeletal structure omits non-essential hydrogen atoms, which helps chemists quickly identify the molecule’s shape and reactivity. Being able to convert Lewis to skeletal structures is crucial for reading and drawing chemical structures in organic chemistry.
