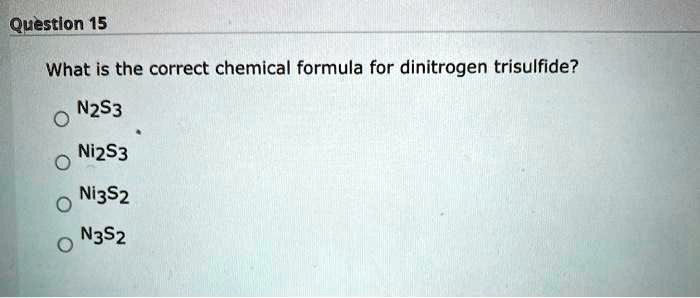
What is the correct chemical formula for dinitrogen trisulfide
The Correct Answer and Explanation is:
The correct chemical formula for dinitrogen trisulfide is N₂S₃.
To understand why this is correct, it is important to break down the compound’s name using the rules of covalent (molecular) nomenclature. Covalent compounds consist of nonmetals, and their names reflect the number of atoms of each element using Greek prefixes.
The name dinitrogen trisulfide includes two prefixes:
- Di- indicates two nitrogen atoms.
- Tri- indicates three sulfur atoms.
So, when combining two nitrogen atoms (symbol N) with three sulfur atoms (symbol S), the proper molecular formula becomes N₂S₃.
Let us now analyze why the other options listed are incorrect:
- Ni₂S₃ involves the element nickel (Ni), which is a metal. This is not valid because the name does not include nickel nor does it refer to an ionic compound.
- Ni₃S₂ also uses nickel and has the wrong ratio for sulfur and nitrogen atoms.
- N₃S₂ includes only nonmetals, but the prefix di- for nitrogen is being violated, and the sulfur count is also inconsistent with trisulfide.
Molecular compounds are named using these systematic prefixes: mono-, di-, tri-, tetra-, penta-, and so forth. These help clearly specify the number of atoms present for each element. The names are constructed in the order the atoms appear in the formula. Since nitrogen appears first in N₂S₃, it is named first as dinitrogen, followed by trisulfide for the three sulfur atoms.
Mastering this nomenclature enhances clarity in chemical communication and helps prevent errors when writing or interpreting molecular formulas in fields such as chemistry, pharmacology, and material science.
