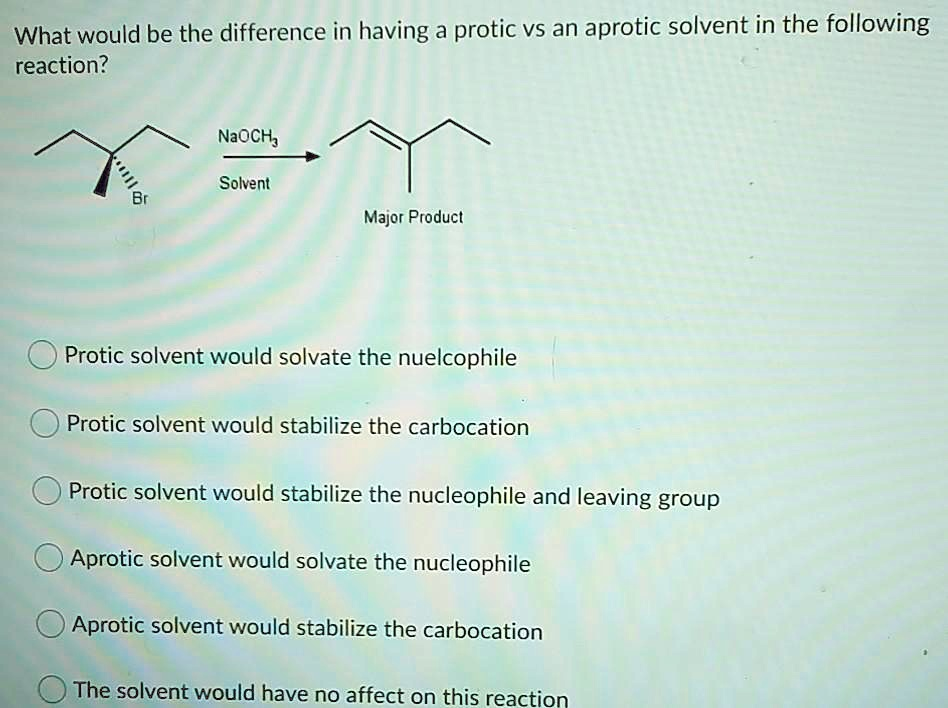
What would be the difference in having a protic vs an aprotic solvent in the following reaction? NaOCH
Solvent Br Major Product Protic solvent would solvate the nucleophile Protic solvent would stabilize the carbocation Protic solvent would stabilize the nucleophile and leaving group Aprotic solvent would solvate the nucleophile Aprotic solvent would stabilize the carbocation The solvent would have no affect on this reaction
The Correct Answer and Explanation is:
The correct answer is: Protic solvent would solvate the nucleophile.
Here’s the explanation:
The reaction shown involves sodium methoxide (NaOCH₃), a strong nucleophile, reacting with a compound bearing a bromine leaving group. This hints at a bimolecular nucleophilic substitution (SN2) mechanism, where the nucleophile directly attacks the electrophilic carbon as the leaving group (Br⁻) departs in one concerted step.
In SN2 reactions, the solvent plays a critical role in controlling the reactivity of the nucleophile. Protic solvents, such as water, methanol, and ethanol, contain hydrogen atoms bound to electronegative atoms like oxygen or nitrogen. These hydrogen atoms are capable of forming strong hydrogen bonds with anionic nucleophiles. When this happens, the nucleophile becomes surrounded or solvated by the solvent molecules. While this stabilizes the nucleophile, it also hinders its ability to attack the substrate efficiently, as the hydrogen-bonded shell must be disrupted before the nucleophile can react.
In contrast, aprotic solvents, such as dimethyl sulfoxide (DMSO), acetone, or acetonitrile, lack hydrogen atoms that participate in hydrogen bonding. They solvate cations like Na⁺ through dipole interactions but leave the nucleophile relatively unsolvated. This “naked” nucleophile is more reactive because it is free to attack the electrophile without solvent interference.
Therefore, if a protic solvent is used in this reaction, it would solvate and stabilize the negatively charged methoxide ion (CH₃O⁻), reducing its nucleophilicity and slowing down the SN2 process. An aprotic solvent would leave the methoxide ion more exposed and reactive, enhancing the reaction rate.
The idea that protic solvents stabilize carbocations or that aprotic solvents affect carbocation stability is relevant to SN1, not SN2. Likewise, saying the solvent has no effect is incorrect. So, the best answer is: Protic solvent would solvate the nucleophile.
