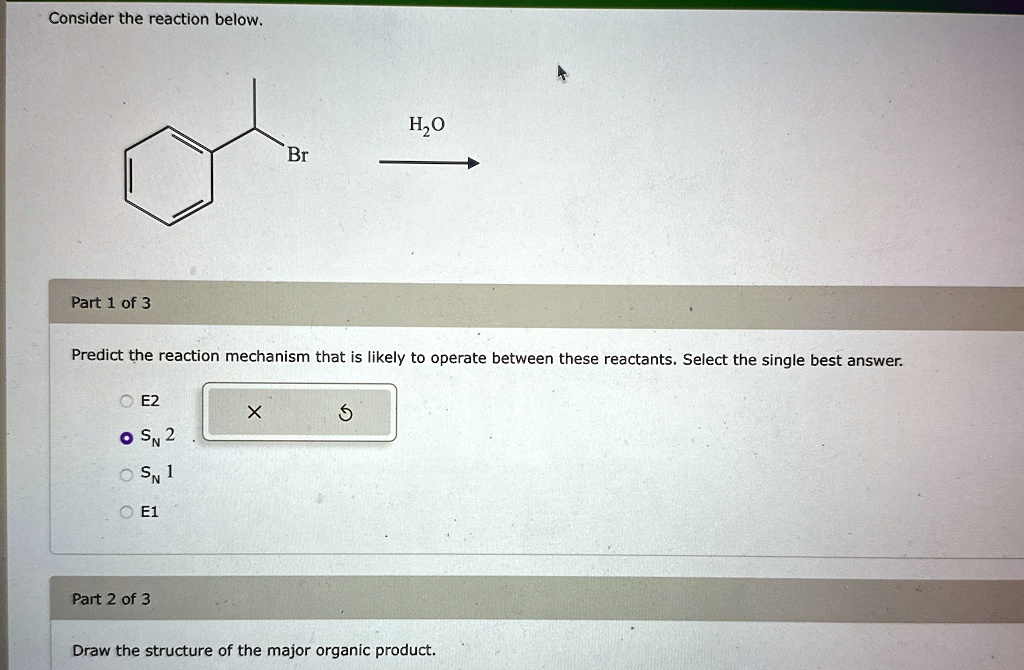
Consider the reaction below. Part 1 of 3
Br Predict the reaction mechanism that is likely to operate between these reactants. Select the single best answer. ? E2 X 5
E1 Part 2 of 3 Draw the structure of the major organic product.
The Correct Answer and Explanation is:
The correct mechanism for the reaction between benzyl bromide and water is SN1, and the major organic product is benzyl alcohol (C₆H₅CH₂OH).
Explanation:
Benzyl bromide is a primary alkyl halide, but what makes it special is the benzyl group’s resonance stabilization. When benzyl bromide reacts with water, the bromide ion (Br⁻) leaves, forming a benzyl carbocation (C₆H₅CH₂⁺). This carbocation is remarkably stable because the positive charge can delocalize over the aromatic ring through resonance. Such stabilization strongly favors an SN1 reaction pathway.
In the first step, the leaving group, Br⁻, departs spontaneously in a slow rate-determining step. This generates the benzyl carbocation. Because this intermediate is stabilized, the energy barrier for its formation is relatively low.
In the second step, the nucleophile (water) attacks the carbocation, forming a protonated alcohol intermediate. Water is both the solvent and the nucleophile in this case, making this an example of solvolysis.
The third and final step involves deprotonation of the protonated intermediate, yielding the neutral product: benzyl alcohol.
This SN1 mechanism proceeds more favorably here than SN2 due to steric accessibility and the unique stability of the intermediate. SN2 reactions typically dominate with primary substrates, but benzyl halides are a key exception due to delocalized stabilization of the intermediate carbocation.
E1 and E2 mechanisms generally require heat and strong bases, which are absent here. Water is a weak base and not a strong enough nucleophile to drive elimination efficiently under mild conditions.
Therefore, the SN1 pathway is most favorable, and the major organic product is benzyl alcohol (C₆H₅CH₂OH).
