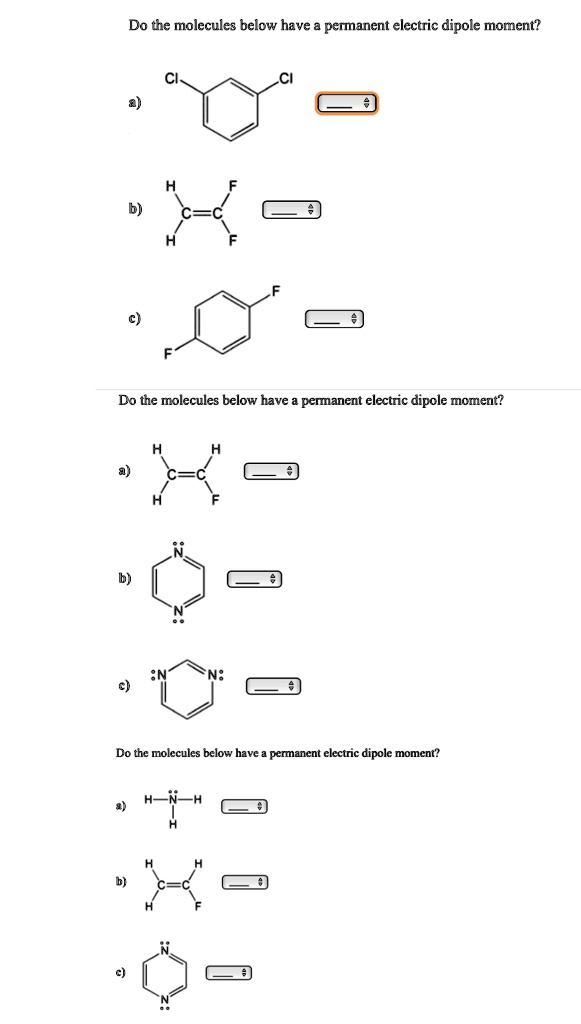
Do the molecules below have permanent electric dipole moment? Do the molecules below have permanent electric dipole moment? Do the molecules below have an electric dipole moment?
The Correct Answer and Explanation is:
To determine whether molecules have a permanent electric dipole moment, we analyze both molecular geometry and electronegativity differences. A permanent dipole arises when there is an uneven distribution of electron density that does not cancel due to the molecule’s geometry. Here’s the evaluation of the molecules in the image:
Set 1:
- a) Monochlorobenzene: Yes. Chlorine is more electronegative than carbon, and since it’s attached to a symmetric benzene ring, its electron-withdrawing nature creates a net dipole along the C–Cl bond that is not canceled.
- b) trans-1,2-Difluoroethylene: No. The fluorines are on opposite sides of the double bond, so their dipoles cancel, yielding no net dipole moment.
- c) Trifluorobenzene (asymmetrical): Yes. If the fluorines are placed asymmetrically on the ring, their individual dipole contributions do not cancel, resulting in a permanent dipole.
Set 2:
- a) trans-1,2-Difluoroethylene (repeated): No, same reasoning as above.
- b) 1,3,5-Triazine (with lone pairs): Yes. The lone pairs on nitrogen create an asymmetric electron cloud distribution that leads to a permanent dipole.
- c) Aromatic triazine with alternating bonds: Yes. The distribution of bonding electrons and resonance contributes to electron asymmetry, maintaining a net dipole.
Set 3:
- a) Diazene (N₂H₂): Yes. The N–H bonds introduce polarity, and the molecular geometry does not cancel the dipole.
- b) Fluoroethylene: Yes. The strong electronegativity of fluorine creates a net dipole because its effect is not offset by the arrangement of the hydrogens.
- c) Aromatic triazine (again): Yes, consistent with set 2c.
In summary, molecules with asymmetrical shape and electronegative substituents generally possess permanent dipole moments. Geometry and electronic structure are key determinants.
