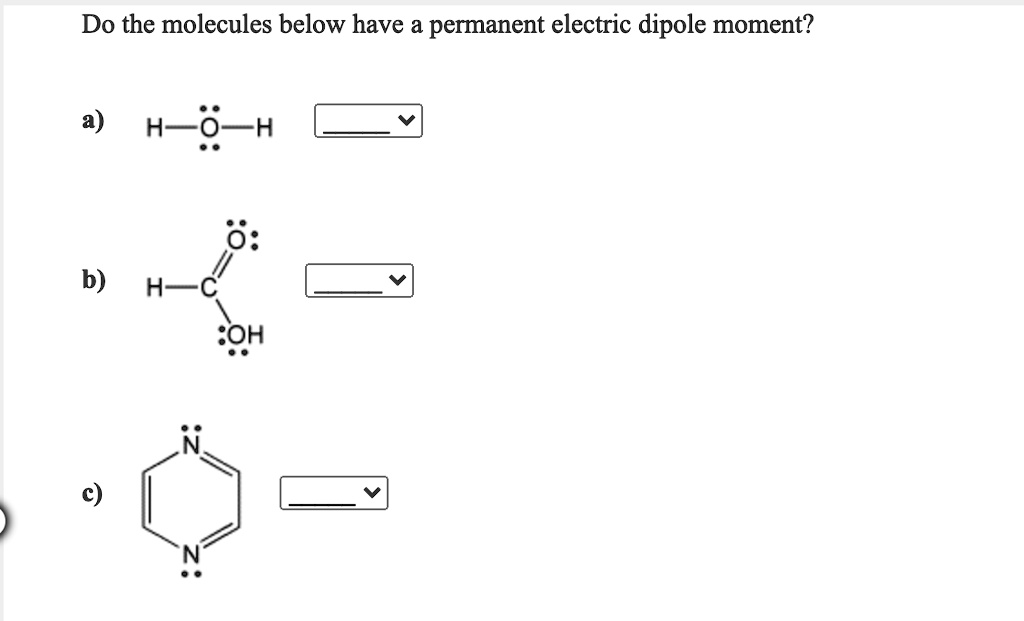
Do the molecules below have a permanent electric dipole moment? H-0-H 0 b) H C :OH
The Correct Answer and Explanation is:
Yes, both molecules—water (H–O–H) and formaldehyde (H–C=O) or methanol with a hydroxyl group (depending on your notation)—have permanent electric dipole moments.
Water (H–O–H): Water has a bent molecular geometry with an angle of approximately 104.5 degrees. The oxygen atom is significantly more electronegative than hydrogen, drawing shared electrons closer to itself and creating a partial negative charge. Each O–H bond is polar, and because the molecule is bent, these bond dipoles do not cancel. Instead, they combine to give a net dipole moment pointing from the hydrogen atoms toward the oxygen. This makes water a classic example of a molecule with a permanent dipole moment. Its polarity is responsible for many of its unusual properties, such as high boiling point and strong cohesion.
Formaldehyde or Methanol Derivative (H–C=O or H–C–OH): Formaldehyde (H–C=O) consists of a carbon doubly bonded to oxygen and singly bonded to two hydrogen atoms. The geometry around the central carbon is trigonal planar, and the oxygen is much more electronegative than carbon. This results in a strongly polarized C=O bond. Although the two C–H bonds are less polar and symmetrically arranged, their dipole contributions do not completely cancel the larger C=O dipole. As a result, formaldehyde has a net dipole moment pointing toward the oxygen atom.
If your structure was intended to represent methanol (H–C–OH), this molecule also has a permanent dipole moment. The O–H bond is polar, and so is the C–O bond. Given the molecular geometry, these dipoles do not cancel, leading to an overall polarity in the direction of the oxygen.
Permanent dipole moments arise when polar bonds are arranged asymmetrically, preventing cancellation of individual dipole vectors. Molecular geometry and electronegativity differences are key in predicting such behavior.
