Elimination The cyclohexane derivative below does not undergo elimination when subjected to E2 conditions. Me Me OTS E2 Conditions No reaction “””Me a) Complete the chair representation of the substrate. One of the substituents has been drawn in for you. Hydrogens may be omitted, but equatorial/axial substituents must be clear! OTS b) In 15 words or fewer, explain why the elimination reaction above does not occur. c) The following E2 elimination reaction will occur, even though it looks (at least on paper) structurally similar to the cyclohexane derivative above. Predict the product, then explain the cause of the difference in E2 reactivities between the molecule below and the cyclohexane derivative above (i.e. why can E2 occur in one instance but not the other?) Explanation: Br NaOMe MeOH d) Another small change to the alkyl halide (seen below) would result in what major product? Br NaOMe MeOH e) After performing the elimination in part d, you find that you (perhaps surprisingly) had a small amount of substitution product as well. Draw that product below, then give an example of a different base you could use to minimize substitution (and maximize elimination). substitution prod. base
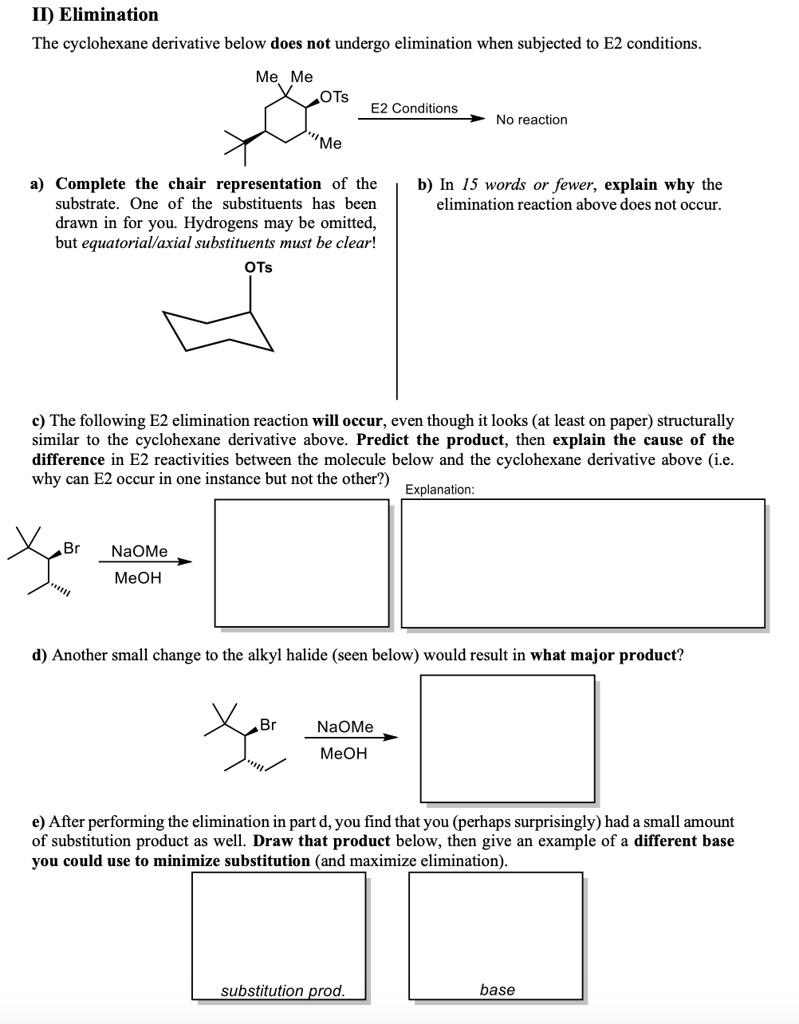
a)
The Correct Answer and Explanation is:
Chair Conformation Completion: The substrate is a substituted cyclohexane with a methyl and OTs group. For E2 to proceed, the OTs (tosylate) and beta-H must be axial and on opposite sides of the ring.
However, the OTS group is equatorial, and the axial conformation would put the methyl group in an unfavorable axial position due to steric hindrance. As a result, the molecule stays in the more stable chair conformation with OTS equatorial, which blocks the required geometry for elimination.
b) Why No Reaction: OTS is equatorial, so no antiperiplanar β-H available for E2 elimination.
c) Why the second molecule does react: In the second molecule (Br instead of OTs), the Br is axial, and there is a trans-diaxial β-H on the adjacent carbon. This correct geometric alignment allows elimination to occur. The product is an alkene formed via E2, where Br is eliminated and a double bond forms.
d) Major Product from Slight Modification: If the compound changes again but keeps Br axial and now adjusts β-H alignment (e.g., via stereochemistry or positioning), the elimination still proceeds. The major product is an alkene with the more substituted (Zaitsev) position—a more stable internal alkene.
e) Substitution Product and Base Choice: Substitution product: the methoxide substitutes for Br, forming a methyl ether. To favor elimination, use a bulky base like tert-butoxide (KOtBu). It hinders backside attack (required for SN2) and promotes E2 by abstracting β-H.
