We can draw three inequivalent Lewis structures for the chlorate ion, ClO3-. The concepts of formal charge and electronegativity can help us choose the structure that is the best representation. 1. Assign formal charges to the elements in each of the structures below. Note: Count oxygen as -2 and hydrogen as +1 from the left for each structure. Structure 1: Cl: 0 O: -1 O: -1 O: -1 Structure 2: Cl: 0 O: 0 O: -2 O: +1 Structure 3: Cl: 0 O: -1 O: -1 O: -1
The Correct Answer and Explanation is:
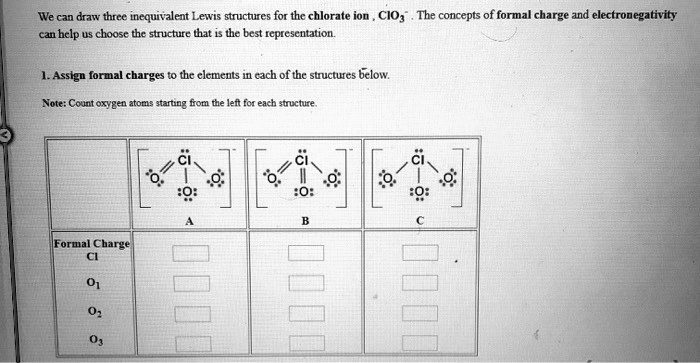
Formal Charges from the Image:
- Structure A:
- Cl: +1
- O (single bonded): -1
- O (single bonded): -1
- O (double bonded): 0
- Structure B:
- Cl: +1
- O (double bonded): 0
- O (single bonded): -1
- O (single bonded): -1
- Structure C:
- Cl: +1
- O (double bonded): 0
- O (single bonded): -1
- O (single bonded): -1
Structures B and C have identical formal charges, while Structure A has three negatively charged oxygens.
Best Structure Justification:
The best Lewis structure minimizes formal charges and places any negative charges on the most electronegative atoms, which in this case are the oxygens.
In Structure A, all three oxygen atoms carry a negative charge, despite chlorine having available d-orbitals to form double bonds and reduce its formal charge. This leads to excessive negative charge on the molecule overall and lacks charge separation.
Structures B and C both delocalize the negative charges better by including one double bond and two single bonds, resulting in two oxygens bearing a charge of -1 and one neutral. The chlorine remains at +1, reflecting the real resonance hybrid.
Although Cl is less electronegative than O, it carries a positive charge in all the structures. But since O prefers to carry negative charge and the overall charge must be -1, Structures B and C more accurately portray this distribution.
Therefore, Structure B or C is the best representation. They offer the lowest formal charge separation consistent with oxygen’s higher electronegativity and are better aligned with resonance stabilization of the chlorate ion.
