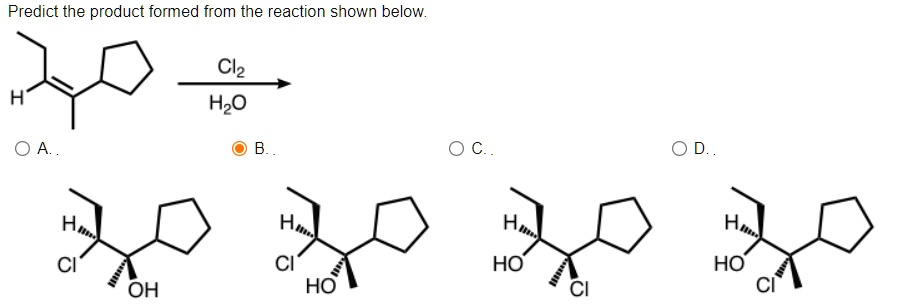
Predict the product formed from the reaction shown below. H Cl
H
O ? ?.. H Cl HO ?.. O C.. OD.. H Cl OH OH Cl H OH Cl
The Correct Answer and Explanation is:
The correct product formed from the reaction shown is option B, which features chlorine and hydroxyl (OH) groups added across the double bond in an anti stereochemical fashion.
This transformation exemplifies a halohydrin formation reaction. When an alkene is treated with aqueous chlorine (Cl₂ in H₂O), the mechanism proceeds via electrophilic addition. Initially, the alkene donates electron density to chlorine, forming a three-membered chloronium ion intermediate. This is a strained, positively charged species in which the chlorine atom bridges the two carbon atoms previously engaged in the double bond.
Water, acting as a nucleophile, then attacks the more substituted carbon of the chloronium ion due to its greater carbocation-like character. This regioselectivity follows principles similar to Markovnikov’s rule. As a result, the OH group is added to the more substituted carbon while Cl attaches to the less substituted one.
The stereochemical outcome is a direct consequence of the backside attack mechanism. Since the nucleophile attacks the chloronium ion from the side opposite to the chlorine, the OH and Cl groups end up on opposite faces of the ring. This leads to anti addition, which is clearly depicted in option B.
Cyclopentane’s rigidity helps preserve the anti relationship in the product, making stereochemistry easily identifiable in this case. All other answer choices deviate either in the regiochemistry of substitution (placing Cl or OH on the wrong carbon) or in the stereochemistry (placing both groups on the same face).
Therefore, option B is the only answer consistent with both the regioselectivity and stereoselectivity expected from halohydrin formation with Cl₂ and H₂O in a cycloalkene context.
