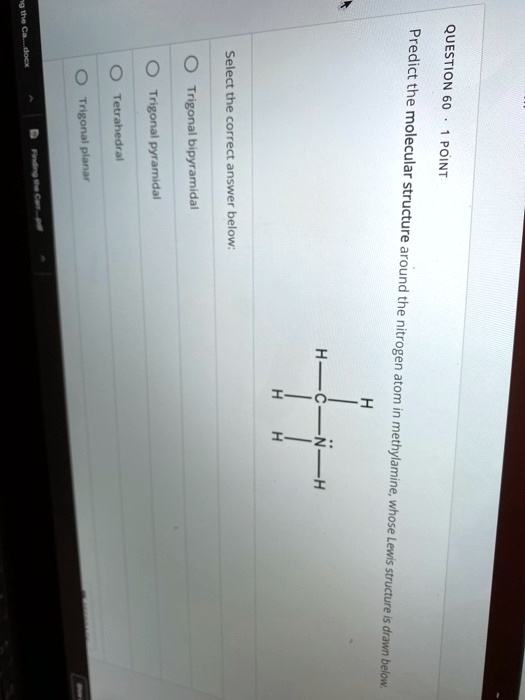
nitrogen atom in methylamine, whose Lewis structure is drawn below. Select the correct answer below: Trigonal bipyramidal Trigonal pyramidal Tetrahedral Trigonal planar H H-C-N: H H
QUESTION 60 · 1 POINT Predict the molecular structure around the nitrogen atom in methylamine, whose Lewis structure is drawn below. Select the correct answer below: Trigonal bipyramidal Trigonal pyramidal Tetrahedral Trigonal planar H H-C-N: H H
The Correct Answer and Explanation is:
The correct answer is Trigonal pyramidal.
To determine the molecular structure around the nitrogen atom in methylamine (CH₃NH₂), we start by analyzing its Lewis structure. The nitrogen atom in methylamine is bonded to two hydrogen atoms and one carbon atom, and it also carries a lone pair of electrons. This gives nitrogen a total of four regions of electron density: three bonding pairs and one lone pair.
Using Valence Shell Electron Pair Repulsion (VSEPR) theory, we understand that these four regions of electron density will orient themselves as far apart as possible. This leads to a tetrahedral electron geometry. However, molecular geometry only considers the positions of the atoms, not the lone pairs.
Because one of the four regions is a lone pair, the molecular geometry becomes trigonal pyramidal. The lone pair occupies more space than bonding pairs, slightly compressing the bond angles between the atoms. This results in a pyramid-like shape with the nitrogen at the apex and the three bonded atoms (two hydrogens and one carbon) forming the base.
It is important not to confuse electron geometry and molecular geometry. While both derive from the arrangement of electron pairs, molecular geometry provides the actual spatial arrangement of atoms. Nitrogen compounds like ammonia (NH₃) and methylamine (CH₃NH₂) share this trigonal pyramidal shape due to their similar bonding environments.
The trigonal bipyramidal geometry only occurs when there are five regions of electron density, which is not the case here. Similarly, tetrahedral molecular geometry requires four bonding pairs and no lone pairs. Trigonal planar would apply only if there were three bonding regions and no lone pairs. Therefore, trigonal pyramidal is the most accurate choice for this structure.
