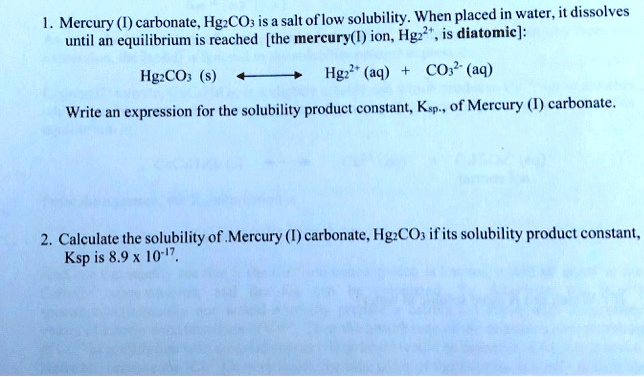
Mercury (II) carbonate, HgCO3, is a salt of low solubility. When placed in water, it dissolves until an equilibrium is reached. The mercury (II) ion, Hg2+, is diatomic. The balanced equation for the dissolution of Mercury (II) carbonate is: HgCO3(s) ⇌ Hg2+(aq) + CO3^2-(aq). Write an expression for the solubility product constant, Ksp, of Mercury (II) carbonate.
The Correct Answer and Explanation is:
1. Solubility Product Expression (Ksp) for Mercury(I) Carbonate
The dissociation of mercury(I) carbonate is given by:
Hg₂CO₃ (s) ⇌ Hg₂²⁺ (aq) + CO₃²⁻ (aq)
Since this is a slightly soluble salt, its solubility product constant (Ksp) is expressed in terms of the concentrations of the dissolved ions at equilibrium. For the above equilibrium, the Ksp expression is:
Ksp = [Hg₂²⁺][CO₃²⁻]
Here:
- [Hg₂²⁺] is the molar concentration of the diatomic mercury(I) ion in solution
- [CO₃²⁻] is the molar concentration of carbonate ion in solution
Because Hg₂CO₃ is a solid, its concentration is not included in the expression.
2. Calculating the Solubility of Mercury(I) Carbonate
Given:
Ksp = 8.9 × 10⁻¹⁷
Let the solubility of Hg₂CO₃ be s mol/L. When one mole of Hg₂CO₃ dissolves, it produces:
- 1 mole of Hg₂²⁺
- 1 mole of CO₃²⁻
So, at equilibrium:
- [Hg₂²⁺] = s
- [CO₃²⁻] = s
Substituting into the Ksp expression:
Ksp = [Hg₂²⁺][CO₃²⁻] = s × s = s²
Therefore:
s² = 8.9 × 10⁻¹⁷
s = √(8.9 × 10⁻¹⁷)
s ≈ 9.43 × 10⁻⁹ mol/L
Explanation
Solubility product constant (Ksp) is an equilibrium constant used for sparingly soluble salts. It reflects the extent to which a compound dissociates into its ions in water. In this problem, mercury(I) carbonate is only slightly soluble. Its dissociation in water produces two types of ions — the diatomic mercury(I) ion (Hg₂²⁺) and the carbonate ion (CO₃²⁻).
The balanced chemical equation shows a 1 to 1 molar ratio for the ions produced. This simplifies the Ksp expression since both ion concentrations are equal at equilibrium. By defining the solubility (s) as the number of moles of Hg₂CO₃ that dissolve per liter of solution, we determine that the concentrations of Hg₂²⁺ and CO₃²⁻ are both equal to s.
Since both ions appear in a 1:1 ratio, the Ksp expression becomes the square of the solubility:
Ksp = s². Solving this equation gives the numerical solubility value.
The very low Ksp value (8.9 × 10⁻¹⁷) confirms that mercury(I) carbonate is highly insoluble in water. The final solubility, 9.43 × 10⁻⁹ mol/L, is also very small, reinforcing the point that only a tiny amount of the salt dissolves before equilibrium is reached.
This method of using the square root of Ksp applies whenever the dissolution produces a 1:1 molar ratio of ions. For salts that produce ions in different ratios (like 1:2 or 2:3), the setup and algebra for solving solubility would require different exponents and adjustments.
