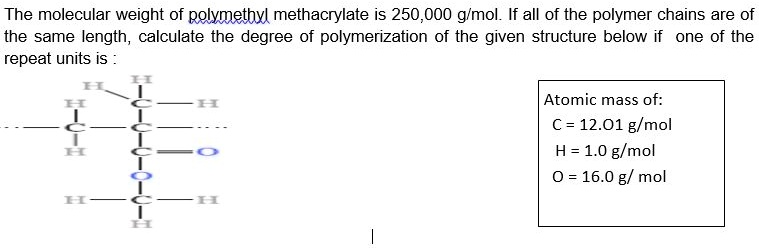
The molecular weight of polymethyl methacrylate is 250,000 g/mol. If all of the polymer chains are of the same length, calculate the degree of polymerization of the given structure below if one of the repeat units is: H H H | | | C—C—C | | | H H O || O | H H C—C | | H H Atomic mass of: C = 12.01 g/mol H = 1.0 g/mol O = 16.0 g/mol
The Correct Answer and Explanation is:
To find the degree of polymerization (DP), we need to calculate how many repeating units make up the entire polymer chain of polymethyl methacrylate (PMMA) with a molecular weight of 250,000 g/mol.
Step 1: Determine the molar mass of one repeat unit
The repeat unit of PMMA (polymethyl methacrylate) is derived from methyl methacrylate, and its chemical formula is:
C₅H₈O₂
Break this down:
- Carbon (C): 5 atoms × 12.01 g/mol = 60.05 g/mol
- Hydrogen (H): 8 atoms × 1.00 g/mol = 8.00 g/mol
- Oxygen (O): 2 atoms × 16.00 g/mol = 32.00 g/mol
Molar mass of one repeat unit = 60.05 + 8.00 + 32.00 = 100.05 g/mol
Step 2: Use the formula for degree of polymerization
Degree of polymerization (DP)=Molecular weight of polymerMolecular weight of repeat unit\text{Degree of polymerization (DP)} = \frac{\text{Molecular weight of polymer}}{\text{Molecular weight of repeat unit}}Degree of polymerization (DP)=Molecular weight of repeat unitMolecular weight of polymerDP=250,000 g/mol100.05 g/mol≈2498.75\text{DP} = \frac{250,000\ \text{g/mol}}{100.05\ \text{g/mol}} \approx 2498.75DP=100.05 g/mol250,000 g/mol≈2498.75
Since the degree of polymerization must be a whole number, we round:DP≈2499\text{DP} \approx 2499DP≈2499
Final Answer:
Degree of polymerization = 2499
Explanation
The degree of polymerization (DP) represents the number of repeating units in a polymer chain. It helps in understanding how long a polymer chain is and directly relates to its physical and chemical properties. For polymethyl methacrylate (PMMA), each repeating unit originates from a methyl methacrylate monomer. This repeat unit has a chemical structure corresponding to C₅H₈O₂.
To calculate the degree of polymerization, the first step is to compute the molar mass of one repeating unit. This involves summing the atomic masses of all atoms in the unit: five carbon atoms, eight hydrogen atoms, and two oxygen atoms. The calculation gives approximately 100.05 g/mol for the repeat unit.
Given that the molecular weight of the entire polymer chain is 250,000 g/mol, we determine how many of these 100.05 g/mol units are present by dividing the total molecular weight by the weight of one unit. This calculation yields about 2498.75 repeating units. Since a polymer cannot have a fraction of a repeat unit, the result is rounded to the nearest whole number, which is 2499.
This means a single PMMA chain with a molecular weight of 250,000 g/mol contains approximately 2499 repeating units of methyl methacrylate. Understanding this relationship is essential in polymer chemistry, as properties like strength, flexibility, and melting point often depend on the degree of polymerization.
