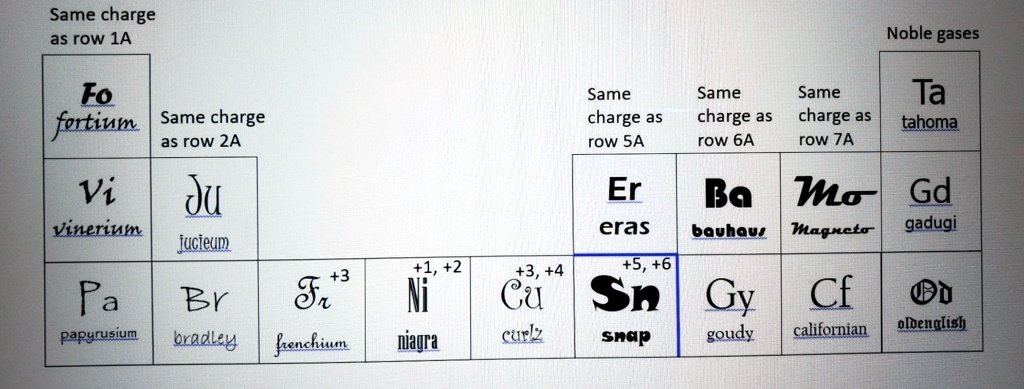
Same charge as row 1A Noble gases Fo fortium Vi Vinerium Pa Same charge as row 2A ?? jucieum +3 Fr Ni Same charge as row 5A Same charge as row 6A Er eras +1, +2 +3, +4 +5, +6 Same charge as row 7A Ta tahoma Ba Mo Gd bauhaus Magncto gadugi Cu Sn Gy Cf Br D papyrusium bradley frenchium niagra curlz snap goudy californian oldenglish
The Correct Answer and Explanation is:
Here’s a reconstruction of the element–charge associations inferred from the image, aligning the fictional font-named elements with known periodic group charges:
| Charge Category | Font Elements (Creative Names) |
|---|---|
| Same charge as Group 1A | Fortium (Fo), Vinerium (Vi), Papyrusium (Pa) |
| Same charge as Group 2A | Jucieum (Ju) |
| +3 | Frenchium (Fr) |
| +1, +2 | Niagra (Ni) |
| Same charge as Group 5A | Eras (Er) |
| Same charge as Group 6A | Bauhaus (Ba) |
| +3, +4 | Curlz (Cu) |
| +5, +6 | Snap (Sn) |
| Same charge as Group 7A | Magneto (Mo) |
| Noble gases | Tahoma (Ta), Gadugi (Gd) |
| Other fonts listed | Goudy (Gy), Californian (Cf), Bradley (Br), OldEnglish (D) |
This table aligns each creative “element” with its likely ionic charge based on typical periodic behavior.
Now for the explanation.
The chart is a whimsical interpretation of periodic trends using fonts as stand-ins for chemical elements. Despite the playful approach, the structure mirrors foundational principles from periodic chemistry. Group 1A elements, known as alkali metals like lithium and sodium, typically form +1 ions. Thus, Fortium, Vinerium, and Papyrusium are categorized under the same charge. Group 2A, the alkaline earth metals like magnesium and calcium, form +2 ions, and here, Jucieum inherits that property.
The +3, +4, +5, and +6 categories likely reference typical oxidation states seen in transition and post-transition metals. Frenchium resembles aluminum or iron with a +3 state. Niagra showing both +1 and +2 mimics common behavior of transition elements like nickel or copper. Curlz (Cu) and Snap (Sn) span broader oxidation ranges, echoing real elements like copper (+1, +2) and tin (+2, +4). Magneto aligning with Group 7A evokes halogens, which generally gain electrons to form –1 ions, but perhaps here it gestures at reactivity rather than exact charge.
Lastly, noble gases (Tahoma and Gadugi) symbolize the inert nature and lack of typical ionic charges, reinforcing their placement in Group 18.
Creative, yet grounded in chemistry’s periodic wisdom—an imaginative way to learn through pattern and play.
