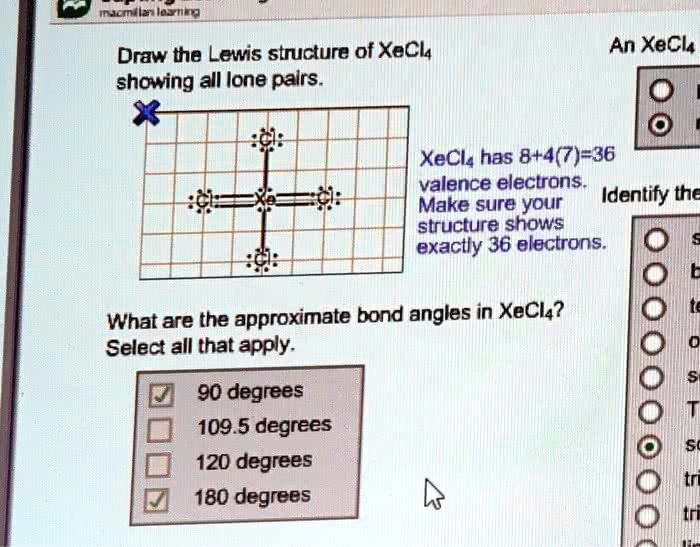
macmillan learning Draw the Lewis structure of XeCl4 showing all lone pairs. An XeCl4 XeCl4 has 8+4(7)=36 valence electrons. Make sure your structure shows exactly 36 electrons. Identify the What are the approximate bond angles in XeCl4? Select all that apply. 90 degrees 109.5 degrees 120 degrees 180 degrees
The Correct Answer and Explanation is:
To draw the Lewis structure of xenon tetrachloride (XeCl₄), begin by accounting for all 36 valence electrons: xenon contributes 8, and each of the four chlorine atoms contributes 7, totaling 8 + 4(7) = 36 electrons.
Step 1: Skeleton Structure Place xenon (Xe) at the center, with four chlorine (Cl) atoms surrounding it. Form single bonds between Xe and each Cl atom, using 8 electrons.
Step 2: Complete Octets Place six electrons (three lone pairs) around each Cl to complete their octets. This uses 4 × 6 = 24 more electrons, bringing the total used to 8 + 24 = 32 electrons.
Step 3: Place Remaining Electrons Now, 36 − 32 = 4 electrons remain. Place these as two lone pairs on the central xenon atom.
The final structure shows xenon with two lone pairs and four bonded chlorine atoms, each surrounded by three lone pairs. The molecular geometry is square planar as predicted by VSEPR theory (AX₄E₂), where the six regions of electron density form an octahedral arrangement but two axial positions are occupied by lone pairs.
Bond Angles in XeCl₄:
- 90 degrees between adjacent Cl atoms in the square plane
- 180 degrees between opposite Cl atoms across the central Xe atom
Thus, the correct bond angles are 90 degrees and 180 degrees. The angles 109.5 and 120 degrees apply to tetrahedral and trigonal planar structures respectively, which do not fit XeCl₄’s square planar shape.
This geometry minimizes repulsion between lone pairs by placing them opposite each other, ensuring that the four Cl atoms lie in a flat plane with symmetric spacing. This elegant arrangement highlights the predictive power of VSEPR theory in rationalizing molecular shape.
