Analyze the IR spectra for salicylic acid (2-hydroxybenzoic acid) and aspirin below and comment on, how would you spectrum that the salicylic acid was converted into aspirin. TRANSRETTANCEI 8 IR of Salicylic Acid 100 OH O OH 4000 3000 200 1500 KAVENUMBER IR of Aspirin 42.65 XT 2922.1 OH -13.23 4000 3500 3000 2500 2000 1752.3 1690.8 1804,6 60% 1000 1012.6 916.3- 840.1 755.4– 704.5- 500 1500 1187.0 1000
The Correct Answer and Explanation is:
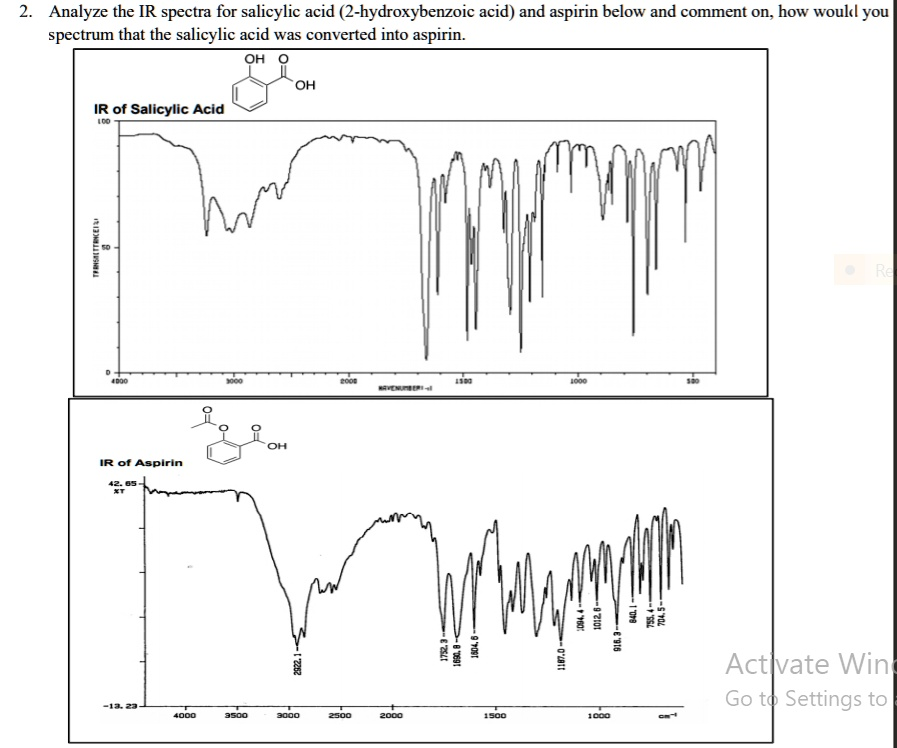
To determine whether salicylic acid has been converted into aspirin, we analyze the infrared (IR) spectra of both compounds and focus on the key differences in their functional groups. Salicylic acid contains both a carboxylic acid and a hydroxyl group attached to the aromatic ring. Aspirin, in contrast, has a carboxylic acid and an ester group. The spectral changes provide clear evidence for this chemical transformation.
In the IR spectrum of salicylic acid, there is a broad O–H stretching absorption between 3200 and 3600 cm⁻¹, which signifies the presence of a free hydroxyl group. There is also a strong C=O stretch around 1690 cm⁻¹, indicating the carboxylic acid carbonyl group. Additionally, O–H bending and C–O stretching bands appear in the 1000 to 1300 cm⁻¹ region.
In the IR spectrum of aspirin, a sharp and intense peak at approximately 1750 cm⁻¹ emerges. This corresponds to the ester C=O stretch, which is not found in the salicylic acid spectrum. The carboxylic acid C=O stretch remains evident near 1690 cm⁻¹. Importantly, the broad O–H stretch in the 3200–3600 cm⁻¹ range becomes significantly weaker or absent. This reduction confirms that the hydroxyl group in salicylic acid has reacted to form the ester linkage in aspirin.
Therefore, the key spectral signature of conversion is the appearance of a new peak near 1750 cm⁻¹ for the ester carbonyl group and the disappearance or weakening of the hydroxyl stretch. These features conclusively support the synthesis of aspirin from salicylic acid by esterification with acetic anhydride. The combined presence of both ester and acid carbonyls in aspirin’s spectrum marks the successful structural modification.
