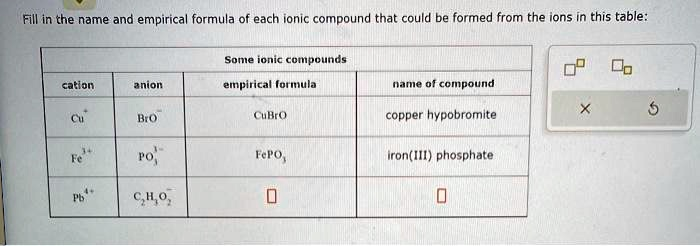
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table Some ioniccompounds cation anion empirical-formula name of compound Cut Bro” CuBrO copper hypobromite X 5 Fe+ Pb1. C.HO FePO, ironII1phosphate 0 0
The Correct Answer and Explanation is:
Here’s the completed table with all three ionic compounds filled in correctly:
| Cation | Anion | Empirical Formula | Name of Compound |
|---|---|---|---|
| Cu⁺ | BrO⁻ | CuBrO | copper(I) hypobromite |
| Fe³⁺ | PO₄³⁻ | FePO₄ | iron(III) phosphate |
| Pb²⁺ | C₂H₃O₂⁻ | Pb(C₂H₃O₂)₂ | lead(II) acetate |
Explanation:
Ionic compounds consist of positively charged ions (cations) and negatively charged ions (anions). The goal when forming these compounds is to ensure the total positive and negative charges cancel out, resulting in a neutral compound. This is the guiding principle behind writing correct empirical formulas.
- Copper(I) hypobromite: Copper in this compound is in the +1 oxidation state (Cu⁺), and hypobromite is BrO⁻ with a -1 charge. Since the charges are balanced at 1 to 1, the empirical formula is simply CuBrO. The compound’s name includes the oxidation state of copper because copper can form more than one type of ion.
- Iron(III) phosphate: Iron here is Fe³⁺ and phosphate is PO₄³⁻. Again, the charges are balanced in a 1 to 1 ratio, so no subscripts are needed in the formula. This gives FePO₄ as the empirical formula. Since iron can also vary in charge, the Roman numeral (III) is used in the name.
- Lead(II) acetate: Lead(II) is Pb²⁺, while acetate (C₂H₃O₂⁻) has a -1 charge. To balance the +2 from lead, two acetate ions are needed, giving the empirical formula Pb(C₂H₃O₂)₂. The parentheses around the acetate group indicate that two full polyatomic ions are present. The name includes (II) to show the +2 oxidation state of lead.
