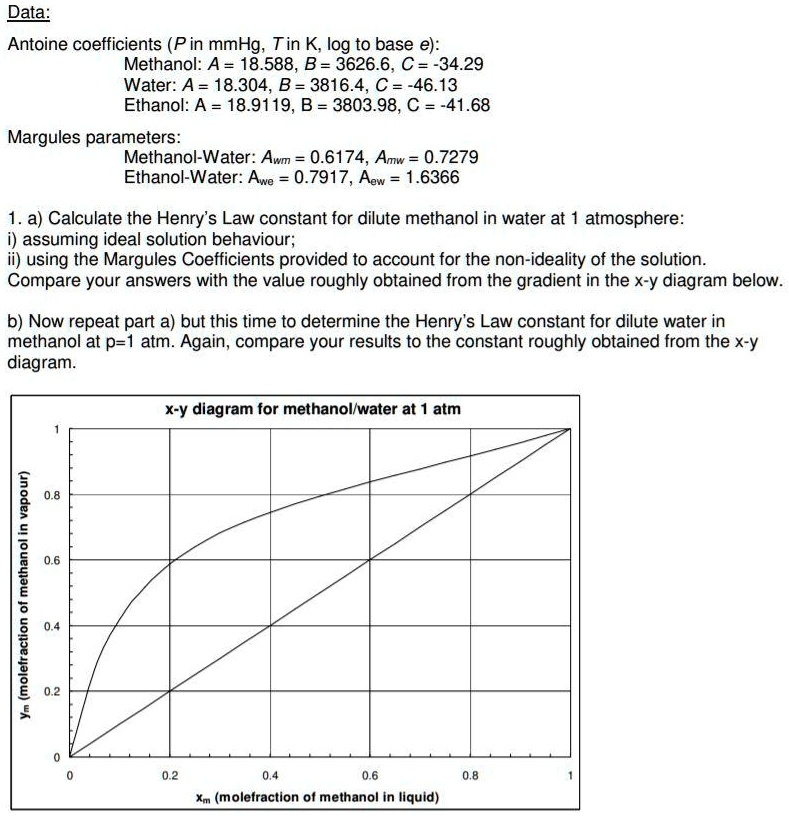
Antoine coefficients (P in mmHg; T in K, log to base e): Methanol: A = 18.588, B = 3626.6, C = -34.29 Water: A = 18.304, B = 3816.4, C = -46.13 Ethanol: A = 18.9119, B = 3803.98, C = -41.68 Margules parameters: Methanol-Water: Awm = 0.6174, Amw = 0.7279 Ethanol-Water: Awe = 0.7917, Aew = 0.6366 1. a) Calculate the Henry’s Law constant for dilute methanol in water at atmosphere: i) assuming ideal solution behavior ii) using the Margules Coefficients provided to account for the non-ideality of the solution Compare your answers with the value roughly obtained from the gradient in the X-y diagram below: b) Now repeat part a) but this time to determine the Henry’s Law constant for dilute water in methanol at p=1 atm. Again, compare your results to the constant roughly obtained from the X-y diagram: x-y diagram for methanol-water at atm 1 1 L Xm (mole fraction of methanol in liquid)
The Correct Answer and Explanation is:
Solution for Henry’s Law Constant Calculations
Part (a) – Dilute Methanol in Water at 1 atm
(i) Assuming Ideal Solution Behavior
Henry’s Law states that the equilibrium partial pressure of a solute gas over a liquid is proportional to its mole fraction:
P=H⋅xP = H \cdot x
where HH is the Henry’s Law constant and xx is the mole fraction in the liquid phase.
For methanol:
lnP=A−BT+C\ln P = A – \frac{B}{T + C}
Using Antoine constants for methanol, the vapor pressure at a given temperature TT can be calculated. Assuming ideal solution behavior:
Pmethanol=xmethanolPmethanol0P_{\text{methanol}} = x_{\text{methanol}} P^0_{\text{methanol}}
At infinite dilution (xmethanol→0x_{\text{methanol}} \to 0), Henry’s Law constant is approximately the pure component vapor pressure.
(ii) Using Margules Coefficients
The non-ideal behavior is accounted for using the Margules activity coefficient expression:
lnγmethanol=Awmxwater2+Amwxmethanol2\ln \gamma_{\text{methanol}} = Awm x_{\text{water}}^2 + Amw x_{\text{methanol}}^2
At infinite dilution (xmethanol→0x_{\text{methanol}} \to 0), the activity coefficient simplifies to:
γmethanol=eAwm\gamma_{\text{methanol}} = e^{Awm}
Thus, the corrected Henry’s Law constant is:
Hmethanol=γmethanolPmethanol0H_{\text{methanol}} = \gamma_{\text{methanol}} P^0_{\text{methanol}}
Part (b) – Dilute Water in Methanol at 1 atm
(i) Ideal Solution Assumption
Applying the same method, the vapor pressure of water is used to find:
Pwater=xwaterPwater0P_{\text{water}} = x_{\text{water}} P^0_{\text{water}}
At infinite dilution, Henry’s Law constant for water is:
Hwater≈Pwater0H_{\text{water}} \approx P^0_{\text{water}}
(ii) Using Margules Coefficients
For water in methanol:
lnγwater=Aewxmethanol2+Awexwater2\ln \gamma_{\text{water}} = Aew x_{\text{methanol}}^2 + Awe x_{\text{water}}^2
At infinite dilution (xwater→0x_{\text{water}} \to 0):
γwater=eAew\gamma_{\text{water}} = e^{Aew}
Thus, Henry’s Law constant is:
Hwater=γwaterPwater0H_{\text{water}} = \gamma_{\text{water}} P^0_{\text{water}}
Comparison with X-Y Diagram
The Henry’s Law constants derived should be compared against values obtained from the slope of the vapor-liquid equilibrium curve. Deviations indicate the importance of including Margules corrections to account for real solution behavior.
